Affiliation:
1Pediatric Department, Privolzhsky Research Medical University, 603950 Nizhny Novgorod, Russian Federation
ORCID: https://orcid.org/0000-0002-2396-5054
Affiliation:
1Pediatric Department, Privolzhsky Research Medical University, 603950 Nizhny Novgorod, Russian Federation
Email: eliseevati@yandex.ru
ORCID: https://orcid.org/0000-0002-1769-3670
Affiliation:
1Pediatric Department, Privolzhsky Research Medical University, 603950 Nizhny Novgorod, Russian Federation
ORCID: https://orcid.org/0000-0002-5961-9794
Affiliation:
2Medical Institute, Peoples’ Friendship University of Russia (RUDN University), 117198 Moscow, Russian Federation
ORCID: https://orcid.org/0000-0002-4961-384X
Affiliation:
3Pediatrics and Child Health Research Institute, Petrovsky National Research Centre of Surgery, 119992 Moscow, Russian Federation
4Pirogov Russian National Research Medical University, 117513 Moscow, Russian Federation
ORCID: https://orcid.org/0000-0003-4861-0919
Affiliation:
5Faculty of Informatics, Mathematics and Computer Science, Direction of Business Informatics, National Research University Higher School of Economics, 603014 Nizhny Novgorod, Russian Federation
ORCID: https://orcid.org/0000-0003-0718-3893
Affiliation:
1Pediatric Department, Privolzhsky Research Medical University, 603950 Nizhny Novgorod, Russian Federation
ORCID: https://orcid.org/0000-0002-6127-5660
Affiliation:
1Pediatric Department, Privolzhsky Research Medical University, 603950 Nizhny Novgorod, Russian Federation
ORCID: https://orcid.org/0000-0002-8531-3174
Explor Med. 2023;4:323–332 DOI: https://doi.org/10.37349/emed.2023.00143
Received: October 14, 2022 Accepted: January 20, 2023 Published: May 30, 2023
Academic Editor: Pietro Vajro, University of Salerno, Italy
Aim: Being overweight and obesity are factors in the negative modification of bronchial asthma (BA). The mechanisms of the aggravating effect of obesity on the course of BA have not yet been fully determined, but include changes in external respiration. The aim of the study was to study the effect of being overweight/obesity on spirometric parameters and on the occurrence of dysanapsis in children and adolescents with BA.
Methods: It was a cross-sectional, open, single-center study. The data were obtained from 428 patients with atopic BA aged 7 years to 17 years, 12.0 [9.0; 14.0], and 72.9% (312/428) of them were boys. The children were divided into 3 groups: group 1—normal body weight; group 2—overweight; and group 3—obesity. All participants underwent spirometry, the ratio of forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) was calculated and the diagnosis of dysanapsis was performed.
Results: As body weight increases, a progressive decrease in FEV1/FVC is revealed—group 1: 79.55% [71.37; 85.43]; group 2: 76.82% [70.12; 82.03]; and group 3: 76.28% [67.04; 79.89] P = 0.004; as well as a decrease in Z FEV1/FVC: group 1—1.23 [–2.18; –0.28]; group 2—1.54 [–2.19; –0.68]; and group 3—1.75 [–2.63; –0.90] P = 0.02. Dysanapsis was detected in 37.7% (159/428) of patients. The incidence of dysanapsis increased statistically significantly with increasing body mass index (BMI) and amounted to: with normal body weight—31.7% (77/243), with overweight—42.0% (55/131), and with obesity—50% (27/54) P = 0.016.
Conclusions: In children and adolescents with BA, as BMI increases, there is a statistically significant decrease in the ratio of FEV1/FVC, and, consequently, bronchial patency; the incidence of dysanapsis also increases statistically significantly. Taken together, this indicates the formation of an obstructive pattern of external respiration under the influence of being overweight and obesity in children and adolescents with BA.
Obesity is considered the accumulation of excess fat, preventing the maintenance of optimal health [1]. It is known that overweight and obesity in children are a factor of negative modification of bronchial asthma (BA) and aggravate its course.
The mechanisms underlying the adverse effects of overweight and obesity on BA have not been fully determined, but are largely due to changes in the functions of external respiration. Modification of spirometric parameters in patients with BA under the influence of being overweight and obesity, as a rule, is formed either by obstructive or restrictive variants.
Restrictive changes in external respiration are characteristic mainly for adult patients with a combined course of asthma and obesity [2]. The restrictive pulmonary pattern found in obese individuals is mainly due to the accumulation of fat in the thoracic and abdominal regions, which leads to a limited downward excursion of the diaphragm and a decrease in the vital capacity (VC), often at normal values of the forced expiratory volume in 1 second/forced VC (FEV1/FVC) ratio.
The obstructive pattern of external respiration in patients with BA and obesity, diagnosed by a decrease in FEV1/FVC, is formed mainly due to dysanapsis and is more common in children and adolescents [3–6]. According to modern concepts, dysanapsis of the respiratory tract is a discrepancy between the volume of the lung parenchyma and the caliber of the respiratory tract, while the volume of the parenchyma is significantly larger than the volume of the respiratory tract [5]. Dysanapsis, therefore, can be considered an important component of the pathogenesis of bronchoobstructive syndrome in patients with BA. Dysanapsis is a relatively independent mechanism of bronchial obstruction formation from type 2 (T2) inflammation and, possibly, causes the formation of the BA phenotype with a low response to basic anti-inflammatory therapy with inhaled glucocorticoids (IGC).
The relationship between dysanapsis and obesity has been demonstrated in epidemiological studies that have shown the effect of excessive weight gain in the first years of life on changes in lung development, namely, an increase in lung volume measured by FVC and a decrease in the diameter of the airways estimated by FEV1, which leads to a reduced ratio of FEV1/FVC and, consequently, the formation of the obstructive pattern of external respiration [7]. These observations suggest that in some children with BA, excessive increase in adipose tissue may affect the development of pulmonary structures [6]. It is also important to study the influence of gender on the formation of dysanapsis, since according to Ripoll and his co-authors [8], there are sex differences in the anatomy of the respiratory tract in children’s patients.
Most studies confirm the prevalence of obstructive patterns of external respiration and dysanapsis in children with BA and overweight/obese [4, 5]. However, there are studies indicating the presence of restrictive changes in external respiration in children with BA and obesity [9]. Thus, at present, the nature of modification of spirometric parameters in children with BA under the influence of being overweight/obesity cannot be considered established. The aim of this study was to study the effect of overweight/obesity on spirometric parameters and the occurrence of dysanapsis in children and adolescents with BA.
The data were obtained from 428 patients with atopic BA aged 7 years to 17 years, boys of 72.9% (312/428) were treated for atopic BA at the 1st Children’s City Clinical Hospital of Nizhny Novgorod, Russia, in 2017–2022. It was a cross-sectional, observational, open, and single-center study.
The criteria for inclusion in the study were:
(1) the diagnosis of asthma established in accordance with the current international conciliation documents [Global Initiative for Asthma (GINA), 2016–2021] by experienced allergists and pulmonologists;
(2) patients aged 7 years and older.
The criteria not inclusion was:
(1) patients with a body mass index (BMI) of less than –1Z of Z value (initially 511 patients were included in the study and 83 of them with a BMI of less than –1Z);
(2) the presence of acute infectious diseases and fever;
(3) systemic use of glucocorticoids.
All patients were assessed with the main anthropometric indicators. All measurements were made without shoes, outerwear, and headgear. Anthropometric parameters (height, body weight, and BMI) were evaluated using tables developed by World Health Organization (WHO), taking into account the gender and age of patients [10].
Calculation of BMI: BMI = body weight (kg)/height (m)2
According to the BMI assessment data in this study, the children were divided into three groups: group 1—normal body weight (BMI values from –1Z to +1Z); group 2—overweight (BMI values above +1Z); and group 3—obesity (BMI values above +2Z). BMI Z-score calculation is performed using a calculator [11].
Spirometric studies were carried out using the MasterScreen Pneumo spirometer (171400-UMR; Jaeger, Germany). When analyzing the spirometry data, the following parameters were evaluated: FVC (L), which reflects the volume of the lungs; FEV1 (L); and FEV1/FVC ratio, which is the main parameter of spirometry for the diagnosis of obstructive disorders. Spirometry data were measured in absolute values and the FEV1/FVC ratio was calculated.
Dysanapsis of the respiratory tract was determined in accordance with the recommendations proposed in the work of Forno et al. [5]. According to them, dysanapsis can be diagnosed if three conditions are met simultaneously:
(1) high or high-normal Z FVC: more than 0.674;
(2) normal Z FEV1: more than –1.645;
(3) low FEV1/FVC ratio: less than 80%.
The FVC and FEV1 Z-score (Z FVC and Z FEV1, respectively) were calculated using the Global Lung Function Initiative calculator, created with the support of the European Respiratory Society (ERS) [12]. The dysanapsis ratio (DR) is calculated using the formula: DR = forced expiratory flow between 25% and 75% of FVC (FEF25–75%)/FVC [13].
The Asthma Control Questionnaire-5 (ACQ-5) was used to quantify the level of BA control. Test scores of less than 0.75 scores corresponded to the controlled, from 0.75 points to 1.5 points—to the partly controlled, more than 1.5 points to uncontrolled asthma [14].
Statistical analysis was carried out using Statgraphics Centurion v.16. Standardized asymmetry and kurtosis were calculated to test the samples for normality. Most of the calculated values were outside the range from –2 to +2, so most of the considered quantitative samples differed from the normal distribution and needed nonparametric statistical tests. The data are presented in the form of median [Q1; Q3], where [Q1; Q3] is the interquartile range—as for indicators with an “abnormal” distribution. The differences between the two groups were determined using Wilcoxon’s W test to compare the medians of the two samples. The Kruskall-Wallis test was used to compare the medians of several groups. Qualitative differences were assessed using the criterion χ2. The differences were considered statistically significant at P < 0.05. The minimum sample size at a significance level of 5% to preserve the statistical power of 80% is 385 participants. A sample of 428 patients is sufficient to identify statistically significant differences in the study of the effect of overweight/obesity on the parameters of external respiration in children with BA.
The analysis was carried out both in the general cohort and separately in boys and girls. The median age of children was 12.0 years [9.0; 14.0], boys and girls were comparable in age (P = 0.79, Table 1). Growth parameters were statistically significantly higher in boys than in girls (P = 0.012), obviously due to sexual dimorphism. At the same time, the BMI in girls and boys was comparable (P = 0.46). It should be noted that none of our patients suffered from morbid obesity (class III obesity).
Comparison of basic characteristics taking into account the gender of patients
| Parameters | Total (N = 428) | Boys (N = 312) | Girls (N = 116) | W | P-value |
|---|---|---|---|---|---|
| Age (years) | 12.0 [9.0; 14.0] | 11.5 [9.0; 14.0] | 12.0 [9.0; 15.0] | 18,400.0 | 0.79 |
| Height (сm) | 153.0 [140.0; 168.0] | 153.0 [140.0; 171.0] | 153.0 [136.5; 164.0] | 15,251.0 | 0.012 |
| Weight (kg) | 47.6 [32.3; 62.0] | 49.0 [35.2; 63.3] | 46.1 [32.5; 59.0] | 16,066.5 | 0.07 |
| BMI (kg/m2) | 19.64 [17.27; 22.66] | 19.70 [17.33; 22.69] | 19.19 [17.22; 22.45] | 17,250.5 | 0.46 |
| BMI Z-score | 0.58 [–0.18; 1.29] | 0.61 [–0.13; 1.33] | 0.44 [–0.33; 1.15] | 15,981.5 | 0.06 |
| ACQ-5 (score) | 0.7 [0.1; 1.4] | 0.7 [0.1; 1.4] | 0.7 [0.2; 1.2] | 4,520.5 | 0.94 |
| FVC (L) | 3.30 [2.60; 4.44] | 3.35 [2.68; 4.61] | 3.11 [2.45; 4.01] | 14,578.0 | 0.002 |
| Z FVC | 1.08 [0.81; 2.09] | 1.05 [0.34; 1.77] | 1.17 [0.46; 2.01] | 19,353.0 | 0.27 |
| FEV1 (L) | 2.50 [1.97; 3.47] | 2.50 [2.02; 3.60] | 2.50 [1.83; 3.20] | 15,814.5 | 0.045 |
| Z FEV1 | 0.15 [–0.74; 0.94] | 0.01 [–0.80; 0.99] | 0.26 [–0.41; 0.87] | 19,450.0 | 0.23 |
| FEV1/FVC (%) | 78.37 [70.80; 83.61] | 77.55 [69.96; 82.96] | 80.59 [74.33; 85.43] | 21,489.5 | 0.003 |
| Z FEV1/FVC | –1.41 [–2.23; –0.54] | –1.34 [–2.28; –0.50] | –1.35 [–2.11; –0.65] | 18,220.0 | 0.91 |
| MEF25% (L/s) | 1.05 [0.69; 1.58] | 1.04 [0.68; 1.60] | 1.06 [0.72; 1.56] | 18,539.0 | 0.70 |
| MEF25% (%) | 59.6 [42.84; 84.27] | 57.69 [41.34; 84.27] | 66.57 [48.51; 84.87] | 19,741.0 | 0.15 |
| FEF25–75% (L/s) | 2.12 [1.52; 3.08] | 2.12 [1.51; 3.11] | 2.13 [1.53; 3.04] | 18,023.5 | 0.95 |
| FEF25–75% (%) | 72.21 [54.84; 91.12] | 69.68 [54.08; 91.12] | 77.15 [62.49; 91.2] | 19,696.5 | 0.16 |
| DR (%) | 0.67 [0.50; 0.84] | 0.65 [0.48; 0.81] | 0.74 [0.58; 0.87] | 21,430.0 | 0.003 |
P < 0.05 represents a significant difference in results. N: number; W: Wilcoxon’s W test; MEF25%: maximal expiratory flow 25% of FVC
The absolute values of FVC (L) and FEV1 (L) were higher in boys than in girls, all P < 0.05. In girls, the percentage ratio of FEV1/FVC and the DR coefficient were higher than those in boys, all P > 0.05. At the same time, the remaining spirometry indicators reflecting bronchial patency: Z FVC, Z FEV1, Z FEV1/FVC, MEF25%, and FEF25–75%, all in absolute and relative values, had no significant sex differences, all P > 0.05. This indicates the comparability of most spirometric parameters reflecting bronchial patency in girls and boys in this sample of patients, but with a tendency to a slightly more pronounced manifestation of the obstructive pattern in boys. There were no differences in the level of control, ACQ-5 patients were comparable (P = 0.9) and corresponded to a partial level of BA control.
It was found that patients with BA who are overweight/obese, compared with patients with normal body weight, have statistically significantly lower values of spirometric parameters reflecting bronchial patency, including percentage of FEV1/FVC (P = 0.004), Z-score FEV1/FVC (P = 0.02), % predicted MEF25% (P = 0.03), and percentage of DR (P = 0.01, Table 2). In addition, there is a tendency to increase Z FVC (P = 0.051) and decrease Z FEV1 (P = 0.90), % predicted FEF25–75% (P = 0.14), however, these differences are not statistically significant.
Comparison of spirometric parameters in patients with BA in patients with normal body weight, overweight, and obesity
| Parameters | Group 1 (normal body weight, N = 243) | Group 2, (overweight, N = 131) | Group 3, (obesity, N = 54) | KWT | P-value |
|---|---|---|---|---|---|
| Z FVC | 0.98 [0.23; 1,75] | 1.11 [0.54; 1.96] | 1.34 [0.57; 2.12] | 5.99 | 0.051 |
| Z FEV1 | 0.18 [–0.75; 0.88] | 0.13 [–0.68; 1.08] | –0.02 [–0.84; 0.99] | 0.22 | 0.90 |
| FEV1/FVC (%) | 79.55 [71.37; 85.43] | 76.82 [70.12; 82.03] | 76.28 [67.04; 79.89] | 11.05 | 0.004 |
| Z FEV1/FVC | –1.23 [–2.18; –0.28] | –1.54 [–2.19; –0.68] | –1.75 [–2.63; –0.90] | 7.50 | 0.02 |
| MEF25% (%) | 64.77 [47.41; 87.86] | 57.62 [37.58; 82.74] | 53.24 [40.43; 76.24] | 7.35 | 0.03 |
| FEF25–75% (%) | 74.04 [56.0; 95.24] | 69.62 [54.51; 91.98] | 69.83 [52.63; 84.09] | 3.97 | 0.14 |
| DR (%) | 0.70 [0.53; 0.87] | 0.64 [0.48; 0.80] | 0.64 [0.46; 0.73] | 8.79 | 0.01 |
P < 0.05 represents a significant difference in results. KWT: Kruskall-Wallis test; N: number
In the group of boys with BA and different BMI, similar patterns were found as in the general group of the studied patients (Table 3).
Comparison of spirometric parameters in patients with BA in patients with normal body weight, overweight, and obesity (boys)
| Parameters | Group 1 (normal body weight, N = 167) | Group 2, (overweight, N = 101) | Group 3 (obesity, N = 44) | KWT | P-value |
|---|---|---|---|---|---|
| Z FVC | 0,95 [0.22; 1,70] | 1.04 [0.45; 1.81] | 1.3 [0.57; 2.23] | 3.55 | 0.17 |
| Z FEV1 | 0.18 [–0.84; 0.98] | –0.09 [–0.75; 0.99] | –0.07 [–0.85; 1.09] | 0.05 | 0.97 |
| FEV1/FVC (%) | 78.91 [70.88; 84.77] | 74.84 [69.59; 81.63] | 74.41 [66.99; 80.06] | 7.66 | 0.02 |
| Z FEV1/FVC | –1.22 [–2.23; –0.27] | –1.64 [–2.29; –0.67] | –1.93 [–2.64; –0.86] | 6.13 | 0.047 |
| MEF25% (%) | 61.85 [44.58; 87.34] | 55.13 [35.09; 79.51] | 54.18 [40.10; 76.81] | 6.45 | 0.039 |
| FEF25–75% (%) | 72.93 [56.00; 93.68] | 62.45 [52.36; 91.98] | 64.43 [53.65; 85.26] | 3.27 | 0.19 |
| DR (%) | 0.68 [0.50; 0.84] | 0.61 [0.44; 0.80] | 0.61 [0.45; 0.72] | 6.34 | 0.042 |
P < 0.05 represents a significant difference in results. KWT: Kruskall-Wallis test; N: number
In girls, there were no statistically significant differences in the parameters of external respiration under the influence of being overweight/obesity, but the direction of differences in spirometric parameters corresponded to those in the general group and among boys (Table 4).
Comparison of spirometric parameters in patients with BA in patients with normal body weight, overweight, and obesity (girls)
| Parameters | Group 1 (normal body weight, N = 76) | Group 2 (overweight, N = 30) | Group 3 (obesity, N = 10) | KWT | P-value |
|---|---|---|---|---|---|
| Z FVC | 1.05 [0.29; 1.91] | 1.49 [0.86; 2.29] | 1.58 [0.58; 1.91] | 4.88 | 0.09 |
| Z FEV1 | 0.19 [–0.47; 0.70] | 0.50 [–0.11; 1.13] | 0.29 [–0.54; 0.87] | 2.99 | 0.22 |
| FEV1/FVC (%) | 81.53 [73.75; 86.35] | 80.34 [74.83; 82.87] | 77.63 [74.62; 78.95] | 2.33 | 0.31 |
| Z FEV1/FVC | –1.28 [–2.14; –0.44] | –1.35 [–2.03; –0.93] | –1.63 [–2.00; –1.56] | 1.98 | 0.37 |
| MEF25% (%) | 67.07 [49.81; 88.00] | 73.59 [47.89; 82.74] | 51.59 [41.07; 71.96] | 2.47 | 0.29 |
| FEF25–75% (%) | 77.16 [56.97; 98.40] | 79.33 [65.95; 88.60] | 73.65 [52.56; 78.53] | 1.47 | 0.48 |
| DR (%) | 0.76 [0.57; 0.93] | 0.74 [0.59; 0.85] | 0.74 [0.58; 0.87] | 1.96 | 0.37 |
P < 0.05 represents a significant difference in results. KWT: Kruskall-Wallis test; N: number
An increase in the proportion of patients with dysanapsis with increasing BMI was revealed (Figures 1–3). In patients with normal body weight, dysanapsis was detected in 31.7% of cases (77/243), in overweight children in 42.0% of cases (55/131), and in obese patients in 50% of cases (27/54, P = 0.016). In the group of boys, the progressive increase in the proportion of patients with dysanapsis with increasing body weight has the character of a trend, in the group of girls it is not statistically significant (P = 0.29).
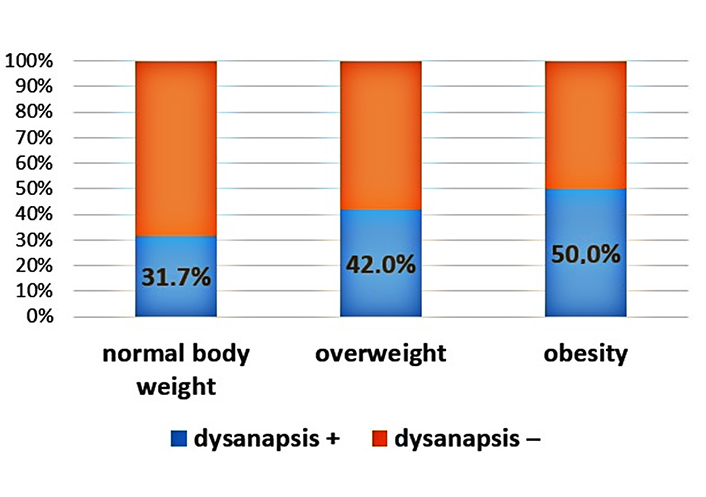
Frequency of occurrence of dysanapsis in patients under the influence of normal body weight, overweight, and obesity (all patients, P = 0.016). Group 1: normal body weight (BMI values from –1Z to +1Z); group 2: overweight (BMI values above +1Z); group 3: obesity (BMI values above +2Z); dysanapsis+: dysanapsis is present; dysanapsis–: dysanapsis is absent
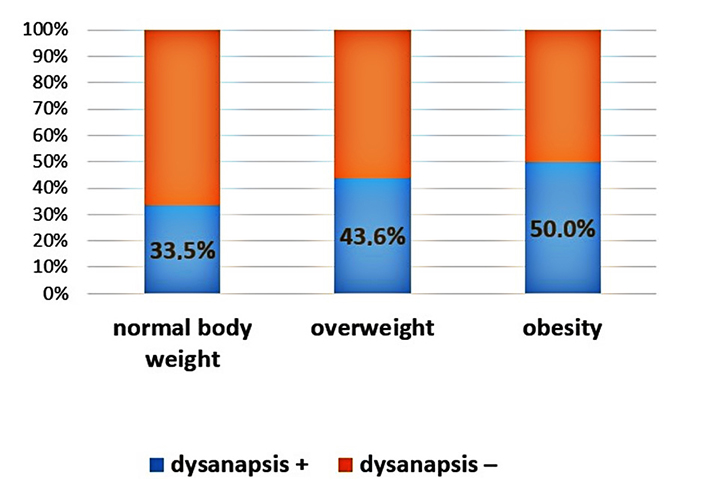
Frequency of occurrence of dysanapsis in patients under the influence of normal body weight, overweight, and obesity (boys, P = 0.07). Group 1: normal body weight (BMI values from –1Z to +1Z); group 2: overweight (BMI values above +1Z); group 3: obesity (BMI values above +2Z); dysanapsis+: dysanapsis is present; dysanapsis–: dysanapsis is absent
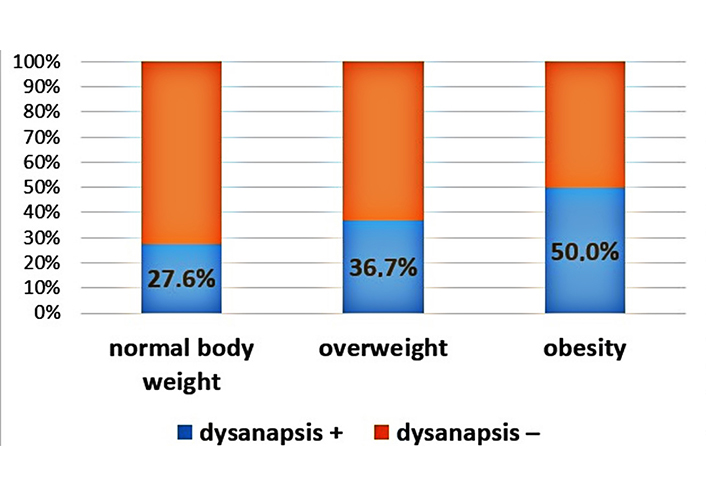
Frequency of occurrence of dysanapsis in patients under the influence of normal body weight, overweight, and obesity (girls, P = 0.29). Group 1: normal body weight (BMI values from –1Z to +1Z); group 2: overweight (BMI values above +1Z); group 3: obesity (BMI values above +2Z); dysanapsis+: dysanapsis is present; dysanapsis–: dysanapsis is absent
In this study, effect of overweight/obesity on spirometric parameters in children with BA was evaluated. The anthropometric parameters of boys and girls in this study were comparable.
We have demonstrated that in children with BA, as BMI increases, the value of the FEV1/FVC ratio decreases, which is currently the leading spirometric parameter in assessing bronchial patency [15]. These patterns were also characteristic of boys and had the character of a trend in the cohort of girls.
Our data are consistent with the results obtained by the research groups Khrisanapant et al. [16], Xu et al. [17], and Han et al. [18], who also note that as BMI increases, the ratio of FEV1/FVC is observed in children and adolescents with BA [19]. The results obtained are also consistent with our pilot study [20]. However, Krol and Litonjua [9] report the presence of restrictive disorders in children and adolescents with BA.
In our study, the median values of Z FVC, reflecting the deviation of the FVC of the lungs from the median population values, were positive in all the studied groups. As the body weight increased, there was a distinct tendency to increase Z FVC: 0.98 [0.23; 1.75], 1.11 [0.54; 1.96], and 1.34 [0.57; 2.12] in patients with normal body weight, overweight, and obese, respectively (P = 0.051). This indicates a low probability of the formation of restrictive changes in external respiration in children and adolescents with a combination of BA and overweight/obesity. On the contrary, the revealed tendencies to increase lung volume as body weight increases create prerequisites for a more frequent formation of dysanapsis in overweight and obese children. The data obtained by us are consistent with the results of Bekkers et al. [21] and Köchli et al. [22], who reported that a higher BMI is associated with an increase in FVC.
At the same time, in Satapathy and his colleagues’ study [23], it was found that the FVC (predicted value) decreases with increasing BMI in children, i.e., the formation of a restrictive pattern of external respiration under the influence of obesity is observed. Perhaps these contradictions with the results obtained by us are due to the fact that in our study there were no children with class III obesity, which may possibly be accompanied by the formation of restrictive changes on the part of the lungs and in children and adolescents.
We found that dysanapsis occurs statistically significantly more often in overweight/obese children than in children with normal body weight. In patients with normal body weight, dysanapsis was detected in 31.7% of cases (77/243), in overweight children in 42.0% of cases (55/131), and in obese patients in 50% of cases (27/54, P = 0.016). Dysanapsis has taken place 42% of cases (55/131) and 50% of cases (27/54), accordingly, compared with children with normal body weight, 31.7% of cases (77/143) of patients (P = 0.016). The data obtained by us are completely consistent with the results of Forno et al. [6], Lang [24], and Jones et al. [25].
The genesis of the increase in the proportion of patients with dysanapsis as body weight increases in patients with BA is currently unclear. Several recent studies have suggested the role of epigenetic programming in obesity and lung development [26–28]. One of the most widely studied epigenetic mechanisms is DNA methylation (DNAm), which is influenced by both genetic factors and environmental influences and which regulates gene expression. It was found that DNAm in specific cytosine-phosphate-guanine (CpG) sites is associated with BMI and lung function. Recent studies have shown that changes in DNA in blood and adipose tissue associated with BMI are primarily a consequence, not a cause, of BMI [29].
The limitation of our study is the lack of analysis of the sexual development of adolescents and the menstrual cycle in girls. In addition, the samples of girls available in the study provides only 70% of the research capacity and dictates the need to continue this study in order to obtain results with a higher level of statistical significance.
Thus, in children and adolescents with BA, under the influence of overweight/obesity, there is a statistically significant decrease in the parameters of external respiration reflecting bronchial patency, as well as an increased incidence of dysanapis, which also forms an obstructive pattern of external respiration in patients. This phenomenon may cause the formation of bronchial obstruction with the inclusion of mechanisms independent of T2 inflammation in patients with BA and a decrease in response to basic anti-inflammatory therapy of IGC.
ACQ-5: Asthma Control Questionnaire-5
BA: bronchial asthma
BMI: body mass index
DR: dysanapsis ratio
FEF25–75%: forced expiratory flow between 25% and 75% of forced vital capacity
FEV1: forced expiratory volume in 1 second
FVC: forced vital capacity
MEF25%: maximal expiratory flow 25% of forced vital capacity
TIE: Conceptualization, Writing—original draft, Project administration. OVK: Methodoly. LAO: Investigastion. EVT: Data curation. RNK: Formal analysis, Validation. GSI: Supervision. VAB: Project administration. DYO: Writing—review & editing. All authors contributed to manuscript revision, read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The study was conducted in accordance with the Helsinki Declaration (2013) and approved by the Ethics Committee of the Privolzhsky Research Medical University No. 13 dated October 10th, 2016.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The datasets for this manuscript are not publicly available because: we are continuing the research, and the datasets will be available as we complete the description.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
