Affiliation:
1Department of Haematology, Alzaiem Alazhari University, Khartoum 11111, Sudan
2Biogenix Molecular Lab, G42 Healthcare, Abu Dhabi 00000, UAE
Email: dia-hassan@outlook.com
ORCID: https://orcid.org/0000-0002-2314-2074
Affiliation:
3Department of Microbiology, Alzaiem Alazhari University, Khartoum 11111 Sudan
ORCID: https://orcid.org/0000-0002-3487-1686
Affiliation:
4Department of Microbiology and Immunology, Military Education Hospital, Khartoum 11111, Sudan
Affiliation:
5Department of Epidemiology, Tropical Medicine Research Institute, National Centre for Research, Khartoum 11111, Sudan
Explor Immunol. 2023;3:406–415 DOI: https://doi.org/10.37349/ei.2023.00109
Received: February 05, 2023 Accepted: April 25, 2023 Published: August 31, 2023
Academic Editor: Joao Santana Silva, Oswaldo Cruz Foundation (Fiocruz), Brazil
Aim: A number of questions remain unanswered concerning how infected individuals regulate their immune response to Plasmodium falciparum (P. falciparum) parasites at varying levels of exposure. Due to the interactions of inflammatory mediators and cytokines with the P. falciparum parasite complex density, several mediators influence parasitaemia and may give some indications of disease severity and represent effective signs in clinical manifestations of malaria disease.
Methods: In this study, various levels of immune response mediators of interleukin 8 (IL-8), tumor necrosis factor-beta (TNF-β, also known as lymphotoxin-α), interferon-gamma (IFN-γ), IL-6, and IL-10 were investigated to the different phases of infection with P. falciparum in hyperendemic states in Sudan (White Nile, Blue Nile). This study vetted the association between certain inflammatory mediators during malaria infection and parasite density. This study was based on a total of 108 cases, in which 86 patients (62.0%) were uncomplicated and (17.6%) were severe, all met the diagnostic criteria and were clinically admitted for malaria infections. Commercial enzyme-linked immunosorbent assay (ELISA) kits were employed to determine the inflammatory mediator’s serum concentration.
Results: The analysis of data indicated that older infected children had substantially raised levels of IFN-γ (P < 0.05), among study groups, levels of IFN-γ, TNF-β, and IL-8 were strongly linked with the severity of malaria, in severe and uncomplicated cases (P < 0.001), IL-6 and IL-10 were significantly associated with severe malaria cases uniquely (P < 0.001). Furthermore, we reported a positive correlation between IL-8 and TNF-β during all infection cases (r = 0.760, P < 0.001). Additionally, in severe malaria cases IL-6 was positively correlated with IL-10 (r = 0.575, P = 0.010).
Conclusions: Eliminating P. falciparum blood-stage infection needs effective, specific, and tuned immune response strategies, which may present in the mediator’s correlations and depend on the density of the infection. Besides the effective levels contribution of certain cytokines that play protective roles during different stages of an infection.
Plasmodium falciparum (P. falciparum), which causes malaria is a leading root of mortality in the tropical countries. African equatorial regions now account for 94% of cases [1]. P. falciparum species present the most prevalent parasite, which infects humans and causes malaria disease throughout the entirety of Sub-Saharan Africa. It is predominant in the East and Horn of Africa, including Sudan, Ethiopia, Djibouti, Eritrea, South Sudan, and Somalia [2]. One of the key reasons that P. falciparum represents a challenging parasite for humans to deal with and impedes the ability of immune responses against plasmodium, is that protective immunity against infection takes several years to acquire, despite recurrent parasite exposure [3, 4], even in high transmission areas. Besides being parasite-specific, naturally acquired immunity is also stage and antigen-specific, so immunity is developed only against erythrocytic stages of infection [5]. The equilibrium of inflammatory cytokine responses, in addition to the interactions of the regulatory mediators, are fundamental to the clearance of Plasmodium parasites, and for preventing serious malaria disease complications during the infection. So as malaria progresses this balance plays a crucial role in eliminating the infection [6].
The cellular activation phase and the systemic response to inflammation are strongly influenced by cytokines, a type of polypeptide derived from cells. It also plays a significant role in mediating inflammation. Several of these factors are multi-functional and have a local or systemic effect [7], and parasites trigger specific immune responses by encouraging peripheral blood mononuclear cells (PBMCs) to trigger the release of cytokines. It is possible that this may be instrumental in activating phagocytic, natural killers, and other effective immune cells, to fight out the liver and blood stages of the parasite [8, 9]. Over the course of the infection by P. falciparum malaria, inflammatory cytokines, and chemokines are raised peripherally in blood and contribute to parasite eradication, and these factors are also believed to be accountable for a variety of symptoms and pathological changes [10]. However, the approaches and processes by which infected people regulate pro/anti-inflammatory responses in consequence of exposure to malaria infection as a result of P. falciparum species are currently incompletely understood.
Studies have established that inflammatory cytokines and regulating mediators are significant predictors of malaria prognosis and determinants of disease severity. And according to some scholars, the balance of anti-inflammatory cytokines interleukin 4 (IL-4) and IL-10 and pro-inflammatory cytokines IL-6, tumor necrosis factor-α (TNF-α), interferon-gamma (IFN-γ), and IL-8 may increase susceptibility to anaemia induced by malaria parasites [11]. Measurement of these cytokines is suggested to determine the parasitaemia rate, clinical severity, symptoms, and outcome [12]. Furthermore, immunity against malaria stages in the blood requires powerful immune response strategies and mechanisms.
Consistent with these prior findings that stated the changes in immune cellular ratios in peripheral blood [13], here the investigation focused on immunological mediators that regulate immune cellular balance. In this study, various levels of cytokine responses were examined in relation to the parasite density consequences of P. falciparum infection, with an emphasis on malaria severity, which is considered to be more severe in children and their immune mediator’s response levels that differ substantially according to previous investigations [14, 15]. According to our hypothesis, the effective response against malaria infection may reflect in forms of associations between the concentration of the inflammatory cytokines, which might explain the protective roles of the inflammatory mediator cells producer during different infection stages and suggest that, before sufficient cytokine responses are elicited, the parasite density must be attained.
Cross-sectional research was carried out in two states, White Nile and Blue Nile which have high endemicity and persistent transmission in the southern region of Sudan, from mid-March to July 2021. The Sudanese patients with P. falciparum infection were the target, infected patients recruited to this study were admitted to National Health Insurance treating centres in both states and those infected individuals with any immunological disorders were excluded. The study population was grouped according to the geographic distribution of infections, age group (adults and children), and sex.
Authorization was obtained from the National Health Insurance Administration, as well as informed permission from the treating physicians, and each volunteer or patient was informed of the purpose of the study. During the study period, 108 patients were enlisted, and blood was collected from all subjects. Five mL of whole venous blood were collected first and then two mL of plasma were separated for serological testing. Individuals in the case group were infected with P. falciparum n = 86 (White Nile 46, Blue Nile 40), while those in the control group were non-infected with P. falciparum n = 20.
The immunochromatography assay has relied on the first detection of Histidine-Rich Protein II (HRP-II) in P. falciparum malaria, and the reference screening criteria were used. Thick blood stains were generated, labelled, and then dyed for 10 minutes with Giemsa stain 10% (pH 7.2), on a clean slide before being studied at ×100 magnification using an optical microscope. Positive results on thick smears are scored using the “plus” system score: if 1 to 9 trophozoites in 100 fields, it reported as +; if 1 to 10 trophozoites in 10 fields, it reported as ++; if 1 to 10 trophozoites per field, it reported as +++; and if more than 10 trophozoites per field, it reported as ++++. Also, the infection by P. falciparum species was verified using thin blood slide smears to the red blood cell-infected per 100 cell fields.
Venous blood from study participants was drawn into Ethylenediaminetetraacetic acid (EDTA) from study participants upon admission to National Health Insurance facilities. Plasma was obtained from blood after centrifugation and kept at –80°C. IL-8, TNF-β, IFN-γ, IL-6, and IL-10 were measured in plasma samples using an automated enzyme-linked immunosorbent assay (ELISA) microplate reader MR-96 (Shenzhen Mindray Bio-Medical Electronics, China) and a competitive sandwich enzyme immunoassay method according to the manufacturer’s instructions (Human IL6 ELISA Kit, Human IL8 ELISA Kit, Human IL10 ELISA Kit, Human TNF-β ELISA Kit, Human IFN-γ ELISA Kit, ELK Biotechnology, Wuhan, China). The detection range of TNF-β, IFN-γ, and IL-8 assays was 15.5–1,000 pg/mL with a sensitivity of 23.6 pg/mL for TNF-β, 5.9 pg/mL for IFN-γ, and 6.7 for the IL-8, while the IL-6 and IL-10 detection range was 7.82–500 pg/mL with a sensitivity of 3.0 pg/mL for IL-6, and IL-10 too. The amounts of all these immune biomarkers were quantified in samples by comparing their optical density (OD) with the reference curves.
Stata version 14 (StataCorp, College Station, Texas) software was used for statistical analysis. To compare continuous variables, Kruskal-Wallis and Mann-Whitney rank sums were used for nonparametric data. Correlations between cytokine profiles were calculated using Spearman’s correlation coefficient. Univariate and multivariate logistic regression models were constructed to assess the coefficients and 95% confidence intervals of interactions between malaria patients and cytokine profiles. Principal component analysis was performed to determine whether cytokine patterns may be utilized to differentiate across children with severe and children with uncomplicated malaria, and healthy groups. In this study, statistical significance was assigned at P < 0.05.
One hundred and eight participants were recruited in this study. The mean age of participants was 3.25 ± 1.81 years (range 1 to 9 years old), with a sex ratio of 1:4 (male:female), and the variations in cytokine levels based on gender were not observed. To assess the relationship between age and cytokine profile in malaria patients, the IFN-γ was significantly higher in the older children group (more than 6 years) compared to the younger children group (P < 0.05). Though IL-6 and TNF-β seem higher in the older group, there is no significant difference among all groups (P > 0.05) (Table 1).
Relationship to age and cytokine profile
| Cytokine | Age group | P-value | ||
|---|---|---|---|---|
< 4 yr old Median (IQR) | 4–6 yr old Median (IQR) | > 6 yr old Median (IQR) | ||
| IL-6 (pg/mL) | 26.0 (17.5–211.0) | 53.0 (18.0–567.0) | 72.5 (25.5–1281.0) | 0.430 |
| IL-8 (pg/mL) | 12.49 (10.19–23.20) | 12.11 (8.50–18.33) | 11.31 (9.25–96.87) | 0.714 |
| IL-10 (pg/mL) | 16.0 (11.0–18.5) | 10.0 (6.0–19.0) | 14.0 (9.25–39.75) | 0.285 |
| IFN-γ (pg/mL) | 1,022.0 (1,003.5–1,187.5) | 1,002.0 (727.0–1,012.5) | 1,036.0 (1,016.5–1,629.75) | 0.007* |
| TNF-β (pg/mL) | 11.0 (7.5–19.5) | 8.0 (7.0–33.0) | 19.50 (6.25–86.0) | 0.762 |
IQR: interquartile range; yr: year. * P-value < 0.05 statistically significantly different
The relationship between cytokine concentrations among study groups was examined (Table 2). There was a significant relationship between severe malaria and cytokine concentrations among patients in comparison to the healthy control group (P < 0.001). The median concentration of IFN-γ was significant (P < 0.001) in severe malaria (111.7 pg/mL) compared to controls (4.0 pg/mL), as in uncomplicated malaria (P = 0.007). TNF-β levels among participants were higher in the two types of clinical malaria compared to controls. It also showed significant relation for severe with control P < 0.001, severe and uncomplicated P = 0.027, and uncomplicated with control P < 0.001. IL-8 levels in the two types of malaria the median was 21.98 pg/mL for severe cases, 11.29 pg/mL for uncomplicated malaria, and (4.0 pg/mL) for controls, showing significant association (P < 0.001). Severe malaria patients had also the highest IL-6 and IL-10 levels (29.0, 18.0 pg/mL), respectively, however, there was no difference between uncomplicated and severe malaria patients (P > 0.05).
Cytokine profile results between matched severe P. falciparum malaria, uncomplicated malaria, and healthy control
| Cytokine | Severe malaria Median (range) | P-valuea | Uncomplicated malaria Median (range) | P-valueb | Control Median (range) | P-valuec |
|---|---|---|---|---|---|---|
| IL-6 (pg/mL) | 29.0 (26.0–214.0) | < 0.001** | 25.0 (17.0–222.0) | 0.338 | 5.50 (3.75–7.0) | < 0.001** |
| IL-8 (pg/mL) | 21.98 (11.64–134.73) | < 0.001** | 11.29 (9.02–17.32) | 0.001* | 4.0 (2.0–5.25) | < 0.001** |
| IL-10 (pg/mL) | 18.0 (10.0–35.0) | < 0.001** | 14.0 (10.0–18.0) | 0.106 | 6.50 (4.0–9.0) | < 0.001** |
| IFN-γ (pg/mL) | 1,117.0 (1,011.0–327.0) | < 0.001** | 1,014.0 (817.0–1,039.0) | 0.007* | 4.0 (3.0–6.0) | < 0.001** |
| TNF-β (pg/mL) | 15.0 (10.0–159.0) | < 0.001** | 9.0 (7.0–19.0) | 0.027* | 2.0 (1.0–3.25) | < 0.001** |
* Significant value as determined by Mann-Whitney rank sum analysis with a level of significance set at P < 0.05; ** highly significant different P < 0.001; a severe versus healthy; b severe versus uncomplicated; c uncomplicated versus healthy
In univariate analysis, the levels of cytokines IFN-γ and IL-8 showed a significant increase in malaria patients compared to controls (P = 0.005; P = 0.003, respectively). Also, multivariate analysis confirmed the considerable alterations in levels of both IFN-γ and IL-8 in malaria patients when compared to control levels (P = 0.008; P = 0.028, respectively) (Table 3).
Association of cytokine profile with malaria patients and control
| Cytokine | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Coefficient | P-value | 95% CI | Coefficient | P-value | 95% CI | |
| IL-6 (pg/mL) | –0.0003147 | 0.568 | –0.0013951 0.0007656 | –0.0007699 | 0.256 | –0.0020986 0.0005588 |
| IL-8 (pg/mL) | –0.0183091 | 0.003* | 0.0060978 0.0305204 | 0.0355878 | 0.028* | 0.0038645 0.067311 |
| IL-10 (pg/mL) | –0.0336167 | 0.054 | –0.0005856 0.067819 | –0.0577672 | 0.192 | –0.1445387 0.0290044 |
| IFN-γ (pg/mL) | –0.0031794 | 0.005* | 0.0009537 0.0054051 | 0.0036688 | 0.008* | 0.0009377 0.0063999 |
| TNF-β (pg/mL) | –0.0009541 | 0.260 | –0.0007051 0.0026133 | –0.000248 | 0.796 | –0.0021277 0.0016317 |
* P-value < 0.05 significantly statistical association. CI: confident interval
For exploring whether cytokines correlate with each other in relation to infection densities, statistical approaches revealed a correlation between the cytokines in both severe and uncomplicated malaria. IL-8 was found to be positively correlated with TNF-β (r = 0.760, P < 0.001) for severe and (r = 0.346, P = 0.004) for uncomplicated (Figure 1). Similarly, a positive correlation was noticed too, between IL-6 and anti-inflammatory cytokines IL-10 (r = 0.575, P = 0.010) in severe malaria patients (Figure 2).
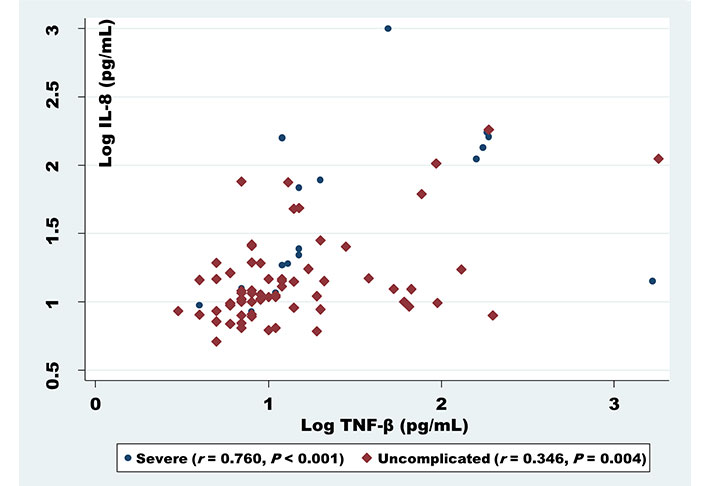
Correlation analysis of IL-8 serum levels with TNF-β among uncomplicated and severe malaria patients
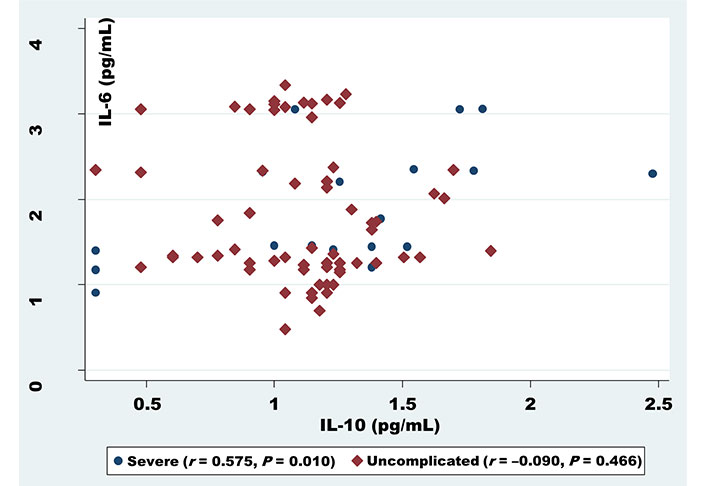
Correlation analysis of IL-6 serum levels with IL-10 among uncomplicated and severe malaria patients
However, during this observation using principal component analysis, it has been found that cytokine profiles were unable to distinguish between children, with either severe or uncomplicated malaria from controls (Figure 3).
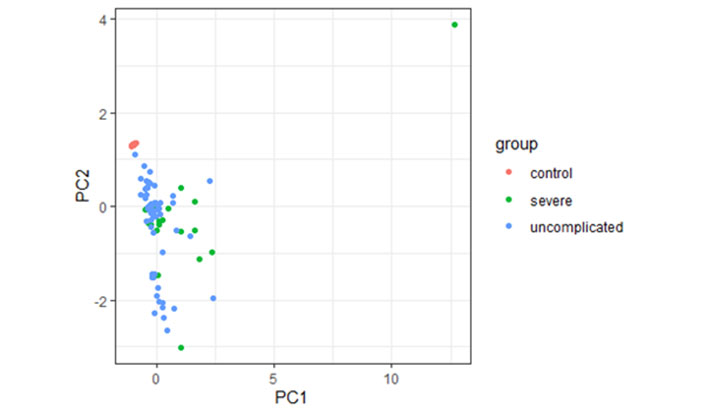
Principal component analysis of cytokine responses in children with severe, uncomplicated malaria patients, and controls. PC1: maximum variation in data; PC2: the second most variation in data
Researchers have spent considerable time and effort studying the essential characteristics and understanding of host immune responses to P. falciparum infections. Despite the complex interactions of the Plasmodium parasite with immune system cells, it is still poorly understood. Cytokine responses significantly influence the emergence and outcome of malaria as separate immunological tactics for infection control. According to the present study, we confirm previous findings of a different pattern of cytokine balance in patients with symptomatic P. falciparum infection [16, 17]. The association of these patterns was also explored to gain a better understanding of immune response strategies and collaboration during malaria infection in an endemic setting.
In this study, individuals infected with P. falciparum malaria expressed significantly higher levels of IFN-γ. A significant relationship was stated between the malaria-infected groups and proinflammatory IFN-γ levels in severe malaria (111.7 pg/mL) and uncomplicated malaria (1,014.0 pg/mL) compared with healthy controls (4.0 pg/mL). And this is consistent with previous research [18]. Furthermore, IFN-γ levels were significantly higher in the older children group (more than 6 years) compared to the younger children group (P < 0.05) [10], implying a protective role for IFN-γ and its association with the rapid killing of P. falciparum, from liver-stage infection through to blood-stage parasite [19].
Additionally, the findings revealed that TNF-β and IL-8, substantially pro-inflammatory cytokines, significantly responded to clinical P. falciparum infection. Compared to non-infected controls, the levels of TNF-β and IL-8 were higher and differed depending on the severity of the infection, during the comparison of the levels as severe and uncomplicated [20, 21]. And these findings illustrate the essential role of TNF-β in immunological development and promoting immune regulation through the innate immune response against P. falciparum infection [22]. In contrast, the pro-inflammatory cytokine IL-8 stimulates neutrophil cells and is an important predictor of malaria parasitaemia, clinical presentation, and mortality [23]. As this study showed, IL-8 levels were significantly associated with malaria intensity.
By using trustworthy biomarkers, it has been discovered that infected people had considerably greater levels of IL-6 and IL-10, confirming the protective effects of the inflammatory cytokines, but neither uncomplicated nor severe malaria patients showed any variations to support the hypothesis that disease tolerance generated by persistent exposure is represented by plasma cytokine concentration in malaria-endemic regions [24].
In studying the correlation between certain cytokines, IL-8/C-X-C motif chemokine ligand 8 (CXCL8), the major mediator involved in the recruitment of neutrophils degranulation and recruitment of inflammatory cells, and TNF-β, a critical regulator that mediates a wide range of immunostimulatory, which shares an effective function in neutrophil activation. In both severe and uncomplicated malaria cases, IL-8 was shown to be positively correlated with TNF-β. This shows an immunological functional link between macrophages and lymphocyte cells during P. falciparum infection, which aids in the elimination of infected red blood cells (RBCs) from circulation [12, 25, 26].
Furthermore, by targeting affected patients with remarkably high plasma concentrations of the regulatory cytokines IL-10, due to parasite density. According to the results, IL-10, a regulatory cytokine, correlated positively with IL-6 an inflammatory cytokine, in malaria cases with high transmission rates [27]. IL-6 has been demonstrated to have double inflammatory properties. A prior study reported an association between IL6, IL10, and parasite density, which supports this finding [28]. It is also evidence that infection tolerance may represent distinct resistance mechanisms for infection clearance depending on disease severity.
The fundamental weakness of our study is its inability to distinguish the influence of previous malaria exposure on the stated age. The variation in cytokine levels production might be explained by the recurrent exposure in older children to malaria infection, which is indicated by the high amount of IFN-γ, IL-6, and TNF-β, and may be because of age-related intrinsic differences in immune system activity [29, 30]. However, by applying principal component analysis, the cytokine patterns were found not to distinguish between children with severe or uncomplicated malaria and the control group.
And the cytokine assessments at a single time point are insufficient to give reliable insights, into the sequence of immune defence tactics, against P. falciparum infection via anti/pro-inflammatory mediator interaction and clinical illness development.
In conclusion, the prevalent results from this study indicated the effective functions of IFN-γ within malaria parasite intensity during the various phases of P. falciparum infection, revealing the levels of contribution of IFN-γ as the key cytokines, which may efficiently impact the clinical outcome of malaria sickness. TNF-β and IL-8, on the other hand, demonstrated a comparable relationship with the severity of infection. Together, IL-6 and IL-10 have preventive functions in severe instances, and they are linked favourably during the infection. Furthermore, these findings provide convenient evidence that IL-8, an important inflammatory cellular recruiter, is correlated with the regulator and neutrophil activator cytokine TNF-β, implying the correlations and the efficient interactions of these cytokines toward parasite intensity. All these data pointed to a double-edged variable protective immune response strategy in eradicating P. falciparum blood stages, influencing illness outcomes, and sharing evidence of the effective involvement of cell mediator makers, namely natural killer (NK) cells, T-cells, and macrophages, which appear to be efficient contributors in host immune response tactics. More research is needed to have a better knowledge of the correlations, interactions, and cytokine patterns associations with P. falciparum infection in an endemic context.
ELISA: enzyme-linked immunosorbent assay
IFN-γ: interferon-gamma
IL-8: interleukin 8
TNF-β: tumor necrosis factor-beta
We send special thanks and appreciation to the National Health Insurance Authority in the White and Blue Nile states for conducting this study in their facilities. Additionally, we thank also the laboratory staff and nurses in National Health Insurance treating centers for their kind help and cooperation. And we wish to express our gratitude to Mr. Mohamed Bala and Dr. Abu Baker M. Alkinani for their expert technical assistance.
DAA: Conceptualization, Writing—original draft, Investigation, Writing—review & editing. M Hussein: Investigation, Validation. M Hassan: Investigation, Validation. AGE: Validation, Visualization, Writing—original draft. MME: Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
This study was approved by the Research and Medical Ethics Committee at Alzaiem Alazhari University, and the Federal Ministry of Health under the Research approval record (MH.CR 2021, M.E.R03021-RN001), and complies with the Helsinki Declaration.
Informed consent to participate in the study was obtained from all participants and parents or guardians of children.
Not applicable.
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
