Affiliation:
1Department of Internal Medicine III, University Hospital Carl Gustav Carus, Technische Universität Dresden, 01307 Dresden, Germany
ORCID: https://orcid.org/0000-0001-7230-4271
Affiliation:
2Department of Diabetology, Madras Diabetes Research Foundation and Dr. Mohan’s Diabetes Specialities Centre, Chennai 600086, Tamil Nadu, India
ORCID: https://orcid.org/0000-0002-4843-1374
Affiliation:
2Department of Diabetology, Madras Diabetes Research Foundation and Dr. Mohan’s Diabetes Specialities Centre, Chennai 600086, Tamil Nadu, India
ORCID: https://orcid.org/0000-0001-5038-6210
Affiliation:
1Department of Internal Medicine III, University Hospital Carl Gustav Carus, Technische Universität Dresden, 01307 Dresden, Germany
Email: charlotte.steenblock@uniklinikum-dresden.de
ORCID: https://orcid.org/0000-0002-9635-4860
Affiliation:
1Department of Internal Medicine III, University Hospital Carl Gustav Carus, Technische Universität Dresden, 01307 Dresden, Germany
3School of Cardiovascular and Metabolic Medicine and Sciences, Faculty of Life Sciences & Medicine, King’s College London, WC2R 2LS London, UK
ORCID: https://orcid.org/0000-0002-5211-2536
Explor Endocr Metab Dis. 2024;1:16–26 DOI: https://doi.org/10.37349/eemd.2023.00004
Received: March 21, 2023 Accepted: August 13, 2023 Published: April 01, 2024
Academic Editor: Victor Gault, Ulster University, UK
Artificial intelligence (AI) has gained attention for various reasons in recent years, surrounded by speculation, concerns, and expectations. Despite being developed since 1960, its widespread application took several decades due to limited computing power. Today, engineers continually improve system capabilities, enabling AI to handle more complex tasks. Fields like diagnostics and biology benefit from AI’s expansion, as the data they deal with requires sophisticated analysis beyond human capacity. This review showcases AI’s integration in endocrinology, covering molecular to phenotypic patient data. These examples demonstrate AI’s potential and power in research and medicine.
We are currently witnessing a revolution in artificial intelligence (AI), nearly affecting and shaping every aspect of modern life. In particular, the health sector is undergoing unprecedented change with the emergence of AI technology. Recently, AI has been progressively used in many fields of medicine, integrating knowledge and data with computer science. In basic terms, AI involves computational approaches in which an algorithm/machine performs a simulation of processes mimicking the cognitive functions of the human brain. Apart from applying the already existing knowledge through an interface between humans and other programs, these algorithms can learn. This subcategory of AI is called machine learning (ML), in which the algorithm automatically learns through experience, without being explicitly programmed for those tasks. The learning process involves adjusting internal parameters or model structures based on the input data, allowing the system to improve its performance over iterations. In this way, the machine is interacting with its environment intelligently and evolves to create more accurate decisions.
How will endocrinologists and diabetologists benefit from these new developments? In many ways, the field of hormone regulation and metabolism is predestined to exploit the power of this technology [1]. Nevertheless, AI will not be required in simple, straightforward clinical or management decisions.
As opposed to other fields of medicine, endocrinology is not linked to one organ structure, but to a complex biological system of hormones and metabolites. Hormones are embedded in an intricate and complex network of local and distal actions. This includes a variety of receptors, signaling pathways, and complicated feedback mechanisms. Thus, a myriad of cellular and hormonal models, with multiple physiological and disease-related interactions exists. These multi-layered and interconnected systems are clearly beyond the comprehension and reasoning of the human brain. Proper endocrine and metabolic regulation exists on a micro and macro cosmos of circadian, circalunar, and circannual rhythms. Both the mechanisms of clock gene-dependent biological rhythms in hormone regulation as well the mechanism of distorted autonomous hormone production in endocrine modules remain poorly understood. It is expected that this remarkable heterogeneity and complexity will be eminently suited to be tackled by AI algorithms [2].
On the other hand, advancing towards more comprehensive paradigms will present greater challenges and higher expectations in generating valuable and practical AI applications. In this context, a subjective evaluation of AI’s current potential in shaping endocrine and metabolic tools for clinical practice is provided.
For the time being one may categorize three different levels of usefulness and availability of AI for the endocrine field: a) already established applications for clinical use, b) systems under current intense development, and c) potential future applications with great and fascinating potential that still require validation and improvement.
The initial US Food & Drugs Administration (FDA) approvals of AI-based medical devices for clinical use were granted in 2015–2016. As of July 2023, the count of FDA-approved AI-based medical devices has exceeded 500 [3]. In Europe, medical devices undergo approval through decentralized agencies, but the figures are comparable [4]. Most of these approved medical devices are prevalent in fields such as radiology, oncology, ophthalmology, and general decision-making [3].
Diabetes is the most common endocrine disease; especially type 2 diabetes (T2D) is affecting almost 10% of the global population; a number which is expected to exponentially rise within the next 20 years [5]. Early detection of T2D can efficiently lead to the prevention of additional complications, and halt the damage caused by this disease [6, 7]. In that regard, ML has shown its efficiency in predicting whether patients will develop T2D [8, 9], but also the risk of potential complications [10]. Different subtypes of T2D have also been recognized in White Europeans [11] as well as in South Asians [12] using data-driven cluster analysis. Similarly, the risk of gestational diabetes and the necessity of intervention can be assessed with ML [13], although further validation will be needed for widespread use.
Diabetic retinopathy (DR) is a frequent macrovascular complication of diabetes. Given the increase of the diabetes pandemic, combined with the prevalence of diabetes, early detection of treatable DR is essential to avoid an overwhelming morbidity and disease burden including blindness in the growing number of people with diabetes around the world. Fortunately, recent developments in diagnostic technologies facilitated the screening for retinal conditions [14]. These efforts include mobile and rural programs in telemedicine, which are now available to take this challenge [15].
ML systems have proven to be effective and accurate in the detection of DR from digital photographs or optical coherence tomography [16, 17]. Several companies to this day have been mushrooming and providing new AI-driven systems. Therefore, AI techniques with high accuracy and efficiency are currently being tested for diagnosing and screening early disease stage DR [16]. More recently convolutional neural network (CNN) algorithms were able to identify even ungradable images in a DR telemedicine screening program [18]. Therefore, in patients with diabetes, images taken during a primary case allow an accurate assessment of the gradeability of non-mydriatic retinal images [18]. This may revolutionize the urgently needed efficiency of DR screening programs enabling prompt-of-care identification of poor-quality images in rural areas and developing countries. AI technologies such as EyeArt and IDx-DR have been approved and are widely used for screening patients for DR (Table 1).
FDA-approved AI devices for endocrine diseases
| Device name | Disease | Application |
|---|---|---|
| AmCAD-UT | Thyroid cancer | Anomaly screening |
| EyeArt | Diabetes | DR screening |
| IDx-DR | Diabetes | DR screening |
| Guardian Connect | Diabetes | Continuous glucose monitoring |
| DreaMed™ Advisor Pro | Diabetes | Monitoring diabetes symptoms |
| MiniMed™ 780G System | Diabetes | Automatic glucose delivery |
| FerriSmart Analysis System | Haemochromatosis | Liver iron concentration estimation |
Another classical domain where telemedicine and digital surveillance can contribute majorly is related to blood glucose monitoring. Despite the more widespread use of insulin pumps and continuous glucose monitoring devices, a majority of individuals with T1D fail to achieve adequate glycemic control [19]. Recent clinical trials showed that insulin dose optimization using an automated AI-based decision support system is effective in adolescents with T1D [20]. An FDA-approved device for managing glucose levels in patients with diabetes is the DreaMed Advisor Pro (DreaMed), which, when used with the MiniMed™ 780G System (Medtronic), provides automatic insulin delivery based on requirements. Another tool, Guardian™ Connect (Medtronic), offers continuous glucose monitoring for patients with diabetes, providing real-time glucose level monitoring and alerts on mobile devices (Table 1). The integration of fully automated insulin delivery systems and AI-based glucose management tools holds the promise of reducing diabetes complications while enhancing and simplifying glycemic control for patients [21].
AI-based applications are also being used in other areas of endocrinology. The FerriSmart Analysis System (FerriScan) has been developed and approved for assessing the concentration of iron in the liver. Furthermore, AmCAD has developed several FDA-approved AI technologies, such as AmCAD-UT, which can analyze the thyroid and detect nodules and potential malignancies (Table 1).
AI-based technologies have demonstrated their effectiveness in various aspects of endocrine disease management, and numerous applications are currently in development, showing promising results pending approval.
For example, AI has shown success in accurately diagnosing common tumors and even distinguishing between tumors within the same organ but with different tissue origins [22–24]. Likewise, AI is now tested and refined for the assessment of thyroid nodules, lymph nodes, and cytopathology specimens [25]. One such approach involves a multi-feature integration model based on ML, enabling the prediction of central lymph node metastasis in papillary thyroid cancer [26]. Such a CNN prediction model may provide a reference for the clinical diagnostics and treatment of papillary thyroid cancer [26].
Similarly, AI is being proposed for diagnosing adrenocortical adenomas based on tissue micro RNA (miRNA) expression [27]. Additionally, the expression of cancer stem cell markers may be used to predict the effectiveness of immune checkpoint inhibitors in treating adrenocortical carcinomas [28].
Furthermore, AI can support clinical teams in pre- and post-operative decision-making [29]. However, the practical and routine implementation of AI in endocrine pathology requires further validation to demonstrate reliability, effectiveness, and real-world utility [25].
Most endocrine diseases have a genetic background [30, 31]. ML can predict the development of T2D and assess the risk of potential complications in affected patients. In cases where endocrine disease has a genetic basis, ML aids in early detection and expedites treatment [32]. Studies reveal that ML algorithms can predict T2D cases using genomic data with higher accuracy than human assessments, and when combined with other biomarkers, the accuracy further improves [33, 34]. The same applies to AI in endocrine imaging and hormone profiling [35–37]. AI in these settings is at an early stage and it’s just starting to unveil its potential. Despite being in the early stages of development in these areas, AI shows promise in providing feasible and effective strategies for early detection, characterization, management, and patient follow-up.
AI algorithms rely on having access to substantial data within a specific context. To demonstrate this principle using images and ML, a straightforward classification experiment using 2D phase contrast images was conducted. Here we classified cell types using images of in vitro differentiated R1 mouse embryonic stem cells (SCRC-1011; American Type Culture Collection) into “mesodermal” (positive) and “not mesodermal” (negative) categories (Figure 1A). We followed an established protocol of mesodermal differentiation [38–40] and proceeded with quantitative reverse transcription polymerase chain reaction (RT-qPCR) validation. Our approach involved utilizing the pre-trained ImageNet model of GoogLeNet [41] for feature extraction and algorithm learning and subsequently employing a support vector machine (SVM) for image classification. The algorithm was trained with 36 images of positive and negative examples, while the learning of the algorithm was validated with 18 and 53 images, respectively. Despite the relatively small sample size, the algorithm achieved an accurate distinction of images with a success rate of 0.8, while avoiding overfitting to our dataset (Figure 1B). We enhanced the algorithm’s performance by augmenting the training set of images, a technique commonly used to improve accuracy [42]. Upon examining the cell images, it becomes evident that the falsely classified images are challenging for the human eye (Figure 1C).
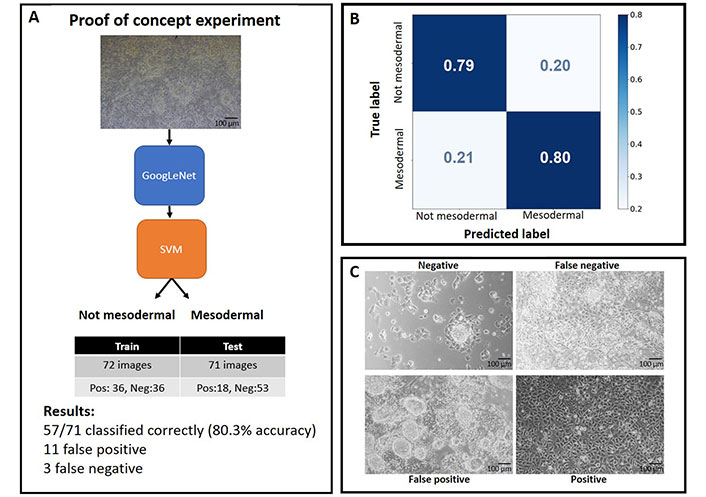
Proof of concept experiment for recognizing cell types based on their morphology from phase contrast images. A. Overview of the algorithm and experimental process; B. confusion matrix generated from the experiment; C. representative examples of images after their classification
Advancing research and enhancing medical care in the field of endocrinology is imperative to better understand, diagnose, and treat endocrine diseases. The emergence of AI-based technologies signals a promising future, as AI holds the potential to aid in achieving this goal. However, the journey to harness AI’s full potential comes with its challenges.
AI systems rely on substantial and high-quality data relevant to their tasks, making data accessibility and privacy concerns crucial. The lack of a specific regulatory pathway for AI-based medical devices in the USA and Europe poses further uncertainties in their approval and oversight. Additionally, AI systems in healthcare often function as clinical decision-support tools, which means their effectiveness relies on the expertise of users and the implementation environment.
One of the most wanted and anticipated applications of ML is in reproductive endocrinology, where it holds promise to improve assisted reproduction outcomes [43, 44]. AI has the potential to enhance fertility through oocyte morphology assessment, computerized semen analysis, tracking folliculogenesis using ultrasonography, determining endometrial receptivity, and optimizing conception based on biological and chemical signatures [44].
ML’s capability for risk identification and primary/secondary prevention holds particular intrigue for endocrine and metabolic diseases, which are often diagnosed late or remain undiagnosed for extended periods. AI algorithms have shown promise in predicting osteoporosis occurrences, screening for hormonal imbalances using electrocardiogram (ECG) monitoring, and offering powerful insights into endocrinology practice. One of the main challenges in the management of osteoporosis is related to diagnostic and therapeutic discrepancies. While the diagnosis is based on bone mineral density detected by dual X-ray absorptiometry, the bulk of early fractures occurs already at non-osteopathic bone mineral density values. Interestingly, recently developed algorithms have been reported to be on par with, or in some cases even surpass, the expertise of clinicians in predicting and evaluating bone quality concerning fracture detection and estimating fracture risk based on imaging and clinical data. These algorithms also demonstrate the potential to devise effective treatment plans [45, 46]. However inadequate reference values may remain the major challenge to generating clinically useful conclusions, even with the best AI technology in place.
Interestingly, there is a striking difference between the expected frequency of disorders such as Conn’s or Cushing’s syndrome and the actual number of patients reported to be treated in various countries. For example, there are 3 times as many patients with Cushing’s syndrome treated in France as in Germany (although Germany has a higher population than France), which could be due to shortcomings in the healthcare system. These potential shortcomings may be related to the decline of active endocrinologists or/and diabetologists in some Western countries. Additionally, other factors can contribute, such as the genetic background of the patients, the accessibility to healthcare, and the expertise of the physicians to recognize minor phenotypic characteristics of these diseases.
We and others have previously reported that even endocrine patients who are exhibiting strong phenotypes such as acromegaly, a tumor-associated symptom, are diagnosed far too late (in our survey, 8 years on average after the first appearance of the characteristic symptoms). Real-time detection of acromegaly from facial images with AI could provide a possible solution to this predicament [47]. Three architectures trained on an ImageNet dataset, namely ResNet50, DenseNet121, and InceptionV3, were used to create a CNN model that could learn to differentiate some images as “healthy” or “acromegaly” [47]. Following the creation of an ensemble model, the system detected acromegaly with high performance through the samples. In the future, the detection of treatable endocrine diseases through face recognition systems, similar to those employed for security purposes at international border control, may become a reality.
Aside from early detection, AI-based technologies have been developed in recent years to improve the remote monitoring of diabetic foot ulcers by employing mobile apps [48]. Diabetic foot ulcers are a growing problem with enormous morbidity and mortality. There is a drastic shortage of foot clinics and they are, if at all, only available in specialized centers. Digitally remote monitoring may help to reduce the need for patient transportation into clinics by guiding the timely and necessary treatment decisions [48].
In what may be a sneak peek into the future, AI algorithms have revealed that commonly used ECG monitoring could provide a way to screen for overt hyperthyroidism or other hormonal levels. Still in the early stages, but this may turn out to be a simple widely accessible, and non-invasive biomarker for this type of disease [49, 50]. This limited selection of examples corroborating the strength of AI for the practice of endocrinology is only a minor glimpse into the power and potential of this technology.
Understanding virtual endocrine patients, molecular signatures, and digital cues by combining ML with dynamic models could revolutionize systems pharmacology and personalized medicine (Figure 2). Although the power of upcoming AI technologies can be both exciting and evoke concern, it’s essential to approach their applications and future with a realistic point of view. As previously mentioned, endocrine systems are usually devised by complex crosstalk between multiple organs and signaling cues. If we would translate this information to an ML context, we can see that the more complex questions we ask the algorithm to answer, the greater the dataset and parameters taken into consideration should be [51]. There is much interest in combining different types of data to have the best results for diagnosis and disease prediction [37, 52–54]. Studies are already making advances in the direction of personalized medicine by taking into consideration the genetic background of patients [55, 56]. These approaches can be unveiled for the background and treatments of endocrine diseases but can raise ethical. There are two naturally occurring conflicts: the potential bias of the algorithm for groups of people and the management of personal data [57]. These problems become even more apparent when it is taken into consideration that the most common problem in ML data analysis is the so-called “black box” [58]. Put simply, it is often unclear how the algorithm arrives at its predictions and conclusions [59]. One way to solve this problem is to pay close attention to the training datasets and their correct labeling and to provide for a standardization process.
Abstract representation of how different inputs (physical, molecular, and digital characteristics) can provide data for AI training. Different combinations of inputs can provide useful outputs, which lead to advances in medicine
From this review, one can observe the plethora of articles and related research in the field of applied ML in endocrinology. This is only part of the clinical research spectrum, and much more is being done at the basic research level which may have clinical applications in the future [60, 61]. More clinicians and researchers will be working with AI or ML-based applications shortly, as they can provide powerful tools to better understand the data generated.
In summary, AI has immense potential to revolutionize various endocrine diseases’ management, diagnosis, treatment, and prognosis. Personalized treatment plans, drug discovery, predictive analytics, and effective telemedicine are among the many benefits AI can bring. While addressing the current challenges in AI-based medical device approval is crucial for equitable access, now is the opportune moment to invest in further research and development of AI-based technologies, paving the way for more efficient and effective healthcare systems in the future.
AI: artificial intelligence
CNN: convolutional neural network
DR: diabetic retinopathy
FDA: US Food & Drugs Administration
ML: machine learning
T2D: type 2 diabetes
ITO: Conceptualization, Investigation, Visualization, Writing—original draft, Writing—review & editing, Validation. RMA and VM: Writing—review & editing. CS: Funding acquisition, Writing—original draft, Writing—review & editing. SRB: Supervision, Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This study was supported by the German Federal Ministry of Education [Clusters4Future SaxoCell, 03ZU1111DA] and by the German Research Foundation DFG, project [314061271] and project [288034826]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
