Affiliation:
Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, Shanghai Institute of Liver Diseases, Shanghai 200032, China
ORCID: https://orcid.org/0000-0003-4579-7925
Affiliation:
Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, Shanghai Institute of Liver Diseases, Shanghai 200032, China
ORCID: https://orcid.org/0000-0003-4432-4122
Affiliation:
Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, Shanghai Institute of Liver Diseases, Shanghai 200032, China
ORCID: https://orcid.org/0000-0003-3494-8636
Affiliation:
Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, Shanghai Institute of Liver Diseases, Shanghai 200032, China
ORCID: https://orcid.org/0000-0002-5419-6315
Affiliation:
Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, Shanghai Institute of Liver Diseases, Shanghai 200032, China
Email: guo.jinsheng@zs-hospital.sh.cn
ORCID: https://orcid.org/0000-0002-9980-8725
Explor Dig Dis. 2023;2:100–117 DOI: https://doi.org/10.37349/edd.2023.00021
Received: January 31, 2023 Accepted: March 20, 2023 Published: June 30, 2023
Academic Editor: Francisco Javier Cubero, Complutense University School of Medicine, Spain
The article belongs to the special issue Drug-induced Liver Injury: From Bench to Clinical Application
Aim: This is a Chinese population-based study aimed to determine the causes and clinical features of drug-induced liver injury (DILI) from traditional Chinese medicines (TCMs) and current Western medicines (WMs) and identify the risk factors of drug-induced liver failure (DILF) and chronic DILI for early recognition and better management.
Methods: The medical records of patients who were diagnosed with DILI for at least six-month follow-up between January 2018 to December 2020 were reviewed and investigated. The risk factors of DILF and chronic DILI were identified by univariate and multivariate logistic regression analysis.
Results: TCMs (47.5%) including herbal medicine (83.0% in TCM-induced DILI) and some Chinese patent drugs were the leading cause of DILI in the present study. Cholestatic type was more associated with severe and chronic DILI. Pre-existing gallbladder disease, initial total bilirubin (TBIL), initial prothrombin time (PT), initial antinuclear antibodies (ANA), and clinical classification are independent risk factors for DILF. Prolonged T0.5AST and T0.5GGT were independent risk factors for chronic DILI [area under the curve (AUC) = 0.812, 95% confidence interval (CI): 0.748–0.876, P < 0.001] with cut-off values of 8.5 days and 29.5 days, respectively.
Conclusions: TCMs especially herbal medicine were the leading causes of DILI, and the risk of developing severe DILI was associated with pre-existing gallbladder disease, clinical classification, initial TBIL, PT, and ANA. T0.5AST and T0.5GGT might serve as indicators for chronicity.
Drug-induced liver injury (DILI) refers to different degrees and types of liver lesions caused by different drugs and (or) their metabolites. DILI is a common adverse drug reaction with a wide spectrum of clinical manifestations. When severe, it may cause acute liver failure (ALF) and even death. A prolonged healing may increase the length of hospitalization and affect the treatment of existing diseases. Thousands of drugs and chemicals including prescribed and over-the-counter drugs, herbal products, nutritional supplements, metals, and toxins have been found to have potential hepatotoxicity [1], and their pathogenic composition and impacts on patients are also diversified with the development of society, medicine, and human cognition. Studies have shown that drugs are the main cause of ALF in the United States [2, 3], Europe [4, 5], and Japan [6]. The annual incidence rate of DILI in Western countries is about 1–20 cases/100,000 people [7, 8]. The annual average incidence rate of DILI in China reported by a recent multi-center retrospective study is about 23.8 cases/100,000 people. Chinese herbal medicine, dietary supplements, and anti-tuberculosis drugs are the main drugs that cause the disease [9].
The clinical symptoms and biochemical indexes of liver function improve in most DILI patients after stopping causative drugs and with hepatic protective remedies, but some patients may progress to drug-induced liver failure (DILF) or develop into chronic liver injury (i.e., chronicity). As reported in the United States and Sweden, about 13–15% of DILI patients progressed to DILF, and the transplantation survival rate of DILF patients was lower than that of ALF induced by other causes [2, 4, 10]. In addition, a minority of acute DILI patients (approximately 3.4–18.9%) may develop chronic liver injury [11]. Pathologically bile duct loss, including vanishing bile duct syndrome (VBDS), and liver cirrhosis were demonstrated in histological examination of these patients.
DILI has always been the research hotspot of liver diseases worldwide. The emergence of new drugs continues to bring clinical problems and challenges. There is a lack of predictive methods for DILF and chronicity in DILI patients. This study collected clinical data of DILI patients hospitalized and followed up in a tertiary hospital in China in the past three years and conducted a retrospective study to investigate the clinical characteristics and causative drugs of DILI. The risk factors of DILF and chronic DILI were specifically analyzed in order to provide reference for the clinical prediction and treatment of DILI.
The study was approved by the ethical committee of Zhongshan Hospital affiliated to Fudan University, Shanghai, China. The study protocol conformed to the provisions of the Declaration of Helsinki. Informed consent was obtained from all subjects. A total of 322 patients diagnosed with DILI who were hospitalized in Zhongshan Hospital from January 2018 to December 2020 were retrospectively analyzed. All patients were followed up for up to 6 months or antecedent death. Roussel Uclaf Causality Assessment Method (RUCAM) score ≥ 6 was the inclusion criteria. All of the competing causes [i.e., group I of acute hepatitis A, hepatitis B, and hepatitis C; biliary obstruction; alcoholism; recent hypotension; and group II of cytomegalovirus (CMV), herpes simplex virus, and Epstein-Barr virus (EBV) infection] were considered when evaluating the RUCAM score of each patient. The patients should have convincing evidence of absent or minimal alcohol consumption, < 15 g alcohol/day for women and < 20 g alcohol/day for men. The exclusion criteria were: i) evidence of liver injury caused by acute viral hepatitis (hepatitis A, B, C, D, and E; CMV; herpes simplex virus; and EBV), convincing evidence of absent or minimal alcohol consumption, < 15 g alcohol/day for women and < 20 g alcohol/day for men, alcoholic liver diseases, autoimmune liver diseases, Wilson disease, bile duct obstruction. The diagnosis of alcoholic liver diseases, autoimmune liver diseases, and Wilson disease were referred to current clinical practice guidelines (https://www.aasld.org/practice-guidelines); ii) complicated with malignant liver tumor and liver cirrhosis; iii) incomplete clinical data, such as unknown medication history and unclear liver function examination before medication; iv) abnormal liver function test before medication; v) pregnancy.
Clinical data of the patients including the following information were recorded: i) basic information of patients: gender, age and comorbidities; ii) clinical manifestations: initial symptoms and latency period (refers to the time interval from the beginning of suspected medication to the first discovery of abnormal liver function examination); iii) type and name of drugs causing DILI, including the components of Chinese herbal medicine and Chinese patent medicine if appliable; iv) laboratory examination: aspartate aminotransferase (AST) and alanine aminotransferase (ALT) which reflect the damage degree of hepatocytes, total bilirubin (TBIL) which reflects liver function of bilirubin metabolism and excretion, albumin (ALB), prothrombin time (PT), and international normalized ratio (INR) of PT which reflect the liver synthesis function, γ-glutamyltransferase (GGT) and alkaline phosphatase (ALP) which are markers of cholestasis. The initial value and peak value of the above indicators, the time interval between TBIL, ALT, AST, ALP, and GGT decreasing from their peak values to half of the peak (T0.5TBIL, T0.5ALT, T0.5AST, T0.5ALP, T0.5GGT); v) all components that impact the RUCAM scale including markers of acute hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), EBV, and CMV infection; vi) serology tests for excluding autoimmune liver diseases such as specific immunoglobulin G (IgG) and immunoglobulin M (IgM), diagnostic autoimmune antibodies of anti-mitochondrial antibody M2 (AMA-M2), antinuclear antibodies (ANA), anti-smooth muscle antibody (ASMA), liver cytosol 1 (LC1), and liver kidney microsome 1 (LKM1); ceruloplasmin test for excluding Wilson disease; vii) auxiliary examination: including abdominal ultrasound, computed tomography (CT), and magnetic resonance cholangiopancreatography (MRCP); viii) treatment: including whether the causative drug had been stopped, and whether liver protective drugs and glucocorticoids had been applied to the patients; ix) outcome: all patients were followed up for at least 6 months from the diagnosis of DILI or antecedent death.
The diagnosis and judgment of causative drugs of DILI were based on RUCAM [12–15]. According to this scale, the causal correlation between drugs and liver injury can be divided into 5 levels: i) highly probable, ≥ 9 points; ii) probable, 6–8 points; iii) possible, 3–5 points; iv) unlikely, 1–2 points; v) excluded, ≤ 0 points. Patients with RUCAM score ≥ 6 or “probable” were included in this study.
According to the DILI judgment standard established by the Council for International Organizations Of Medical Sciences (CIOMS) [14], the clinical classification of DILI was calculated by R value {R = [ALT measured value/ALT upper limit of normal (ULN)]/(ALP measured value/ALP ULN)} and divided into: i) hepatocellular type of injury, ALT ≥ 3× ULN, and R ≥ 5; ii) cholestatic type of injury: ALP ≥ 2× ULN, and R ≤ 2; iii) mixed type of injury: ALT ≥ 3× ULN,ALP ≥ 2× ULN, and 2 < R < 5; iv) abnormal liver biochemical examination type: ALT and ALP were abnormal, but the above criteria were not met. Differences in clinical characteristics of DILI associated with different causative drug were analyzed.
The severity of DILI was defined in grades according to the recent guidelines for diagnosis and treatment of DILI [11]: i) grade 0 (no liver injury): the patient was exposed to causative drugs but no liver injury; ii) grade 1 (mild liver injury): serum ALT and/or ALP recoverably increased, with TBIL < 2.5 ULN, and INR < 1.5; iii) grade 2 (moderate liver injury): serum ALT and/or ALP increased, TBIL ≥ 2.5 ULN, or INR ≥ 1.5; iv) grade 3 (severe liver injury): severe clinical illness with significant jaundice and disabling symptoms, serum ALT and/or ALP increased, and TBIL ≥ 5 ULN, with or without INR ≥ 1.5; v) grade 4 (ALF): the level of serum ALT and/or ALP increased, TBIL ≥ 10 ULN or a TBIL that increased by ≥ 17.1 µmol/L per day, INR ≥ 2.0 or prothrombin activity (PTA) < 40%, or secondary loss of other organ functions, such as the brain (encephalopathy) or kidney (hepatorenal syndrome); vi) grade 5 (lethal): death due to DILI or liver transplantation to survive.
During follow-up, patients with grade ≤ 3 in the follow-up process were classified as the non-DILF group, and patients who progressed to grade ≥ 4 was defined as the DILF group.
According to the clinical guidelines, chronic DILI is defined as the failure to return to normal of biochemical indexes of liver function and/or clinical symptoms such as ascites, hepatic encephalopathy, and portal hypertension within 6 months of onset [14].
The DILI patients were divided into a recovered group and a chronicity group according to whether the liver enzymes, bilirubin, or symptoms of the DILI patients returned to normal within 6-month’s follow-up.
According to the categories of causative drugs, it was divided into traditional Chinese medicine (TCM) and health care product (CM) group, Western medicine (WM) group, and CM + WM group. The clinical manifestation and classifications of liver injury within different categories of causative DILI drugs were compared.
IBM® SPSS® Statistics 25.0 software was used for data analysis. The median with the interquartile spacing was given for continuous variables. Frequencies and percentiles were given for categorical variables. The Mann-Whitney U-test and Kruskal-Wallis test were used for continuous variables, and the chi-squared test and Fisher’s exact test were used for categorical variables. Subsequently, multivariate logistic regression analysis, consisting of variables with P < 0.05 on univariate analysis, was carried out to identify the risk factors independently associated with DILF and chronic DILI. Odds ratios (ORs) with 95% confidence intervals (CIs) of ORs were given. The receiver operating curve (ROC) was used to estimate the predictive value of the time intervals of AST and GGT declining from their peak value to half of the peak (T0.5AST and T0.5GGT, respectively). A P value was considered as statistically significant when it was < 0.05.
As shown in flowchart at Figure 1, a total of 795 patients diagnosed with DILI were searched, including 781 patients with RUCAM score ≥ 3. After further selection by exclusion criteria, three hundred and twenty-two DILI patients were finally included in the analyses.
The demographics and clinical and laboratory characteristics of the 322 patients with DILI are shown in Table 1. Among 322 patients in this study, 196 were female (60.9%) and 126 were male (39.1%). There was no gender difference in liver function indexes at presentation (P > 0.05). The age of patients ranged from 21 years old to 87 years old. When the ages were divided into seven groups from 20 years old to 89 years old with 10 years interval, the majority of patients were distributed within 50 years old to 69 years old (52.2%). The latency period is defined as the time interval from the beginning of medication to the first discovery of abnormal liver function examination. The majority of the DILI patients (252 cases, 78.3%) have a latency of 5–90 days. Gastrointestinal symptoms (such as abdominal discomfort, anorexia, nausea, and vomiting, 146 cases, 45.3%), jaundice (118 cases, 36.6%), choluria (102 cases, 31.7%), and dizziness/fatigue (82 cases, 27%) were the most frequent symptoms at presentation. The common comorbidities were malignant tumors (122 cases, 37.9%), gallbladder disease (111 cases, 34.5%), hypertension (90 cases, 27.9%), and fatty liver (85 cases, 26.4%). In terms of the clinical classification of DILI, 183 cases (56.8%) presented with hepatocellular injury, 23 cases (7.2%) presented with cholestatic injury, and 18 cases (5.6%) presented with mixed type. Another 98 (30.4%) patients can’t be classified by R value and thus grouped in abnormal liver biochemical examination type. Patients with malignant tumors presented more with abnormal liver biochemical examination type (74.5%) while those without malignant tumor showed more with hepatocellular type (80.9%).
Clinical characteristics of 322 DILI patients based on the type of liver damage
| Variable | Clinical types | ||||||
|---|---|---|---|---|---|---|---|
Hepatocellular (n = 183; 56.8%) | Cholestatic (n = 23; 7.1%) | Mixed (n = 18; 5.6%) | Abnormal liver biochemical examination (n = 98; 30.5%) | P value | |||
| Age [M (IQR)] | 55 (43–65) | 62 (49–72) | 56 (47–67) | 60 (49–66) | 0.029 | ||
| Gender [n (%)] | |||||||
| Male | 74 (40.4) | 12 (52.2) | 5 (27.8) | 35 (35.7) | 0.358 | ||
| Female | 109 (59.6) | 11 (47.8) | 13 (72.2) | 63 (64.3) | |||
| Latency (days) | |||||||
| < 5 | 13 (7.1) | 0 (0) | 1 (5.6) | 1 (1.0) | 0.278 | ||
| 5–90 | 141 (77.0) | 19 (82.6) | 13 (72.2) | 79 (80.6) | |||
| > 90 | 29 (15.8) | 4 (17.4) | 4 (22.2) | 18 (18.4) | |||
| Hypertension | |||||||
| Yes | 51 (27.9) | 7 (30.4) | 5 (27.8) | 27 (27.6) | 0.994 | ||
| No | 132 (72.1) | 16 (69.6) | 13 (72.2) | 71 (72.4) | |||
| Diabetes | |||||||
| Yes | 6 (3.3) | 3 (13) | 1 (5.6) | 9 (9.2) | 0.056 | ||
| No | 177 (96.7) | 20 (87) | 17 (94.4) | 89 (90.8) | |||
| Coronary heart disease | |||||||
| Yes | 14 (7.7) | 2 (8.7) | 2 (11.1) | 5 (5.1) | 0.601 | ||
| No | 169 (92.3) | 21 (91.3) | 16 (88.9) | 93 (94.9) | |||
| Gallbladder disease | |||||||
| Yes | 71 (38.8) | 10 (43.5) | 8 (44.4) | 22 (22.4) | 0.024 | ||
| No | 112 (61.2) | 13 (56.5) | 10 (55.6) | 76 (77.6) | |||
| Fatty liver | |||||||
| Yes | 55 (30.1) | 5 (21.7) | 4 (22.2) | 21 (21.4) | 0.439 | ||
| No | 128 (69.9) | 18 (78.3) | 14 (77.8) | 77 (78.6) | |||
| Malignant tumor | |||||||
| Yes | 35 (19.1) | 10 (43.5) | 4 (22.2) | 73 (74.5) | < 0.001 | ||
| No | 148 (80.9) | 13 (56.5) | 14 (77.8) | 25 (25.5) | |||
| Chronic hepatitis B | |||||||
| Yes | 12 (6.6) | 0 (0.0) | 1 (5.6) | 2 (2.0) | 0.266 | ||
| No | 171 (93.4) | 23 (100.0) | 17 (94.4) | 96 (98.0) | |||
| Laboratory parameters | |||||||
| TBIL | 56.8 (18.8–136.3) | 95.8 (15.4–168.9) | 82.8 (22.8–164.7) | 9.0 (6.5–16.8) | < 0.001 | ||
| ALB | 40 (37–44) | 36 (34–42) | 41 (36–43) | 44 (41–46) | < 0.001 | ||
| ALT | 1,024 (496–1,500) | 207 (74–329) | 453 (323–532) | 77 (57–124) | < 0.001 | ||
| AST | 723 (331–1,076) | 126 (71–217) | 229 (185–330) | 54 (39–82) | < 0.001 | ||
| ALP | 138 (88–178) | 388 (223–509) | 281 (178–334) | 90 (63–131) | < 0.001 | ||
| GGT | 174 (91–278) | 318 (146–543) | 300 (165–722) | 49 (29–86) | < 0.001 | ||
| PT | 12.2 (11.4–13.4) | 11.5 (11.0–12.1) | 11.2 (10.7–12.2) | 11.1 (10.6–11.7) | < 0.001 | ||
| INR | 1.07 (1.01–1.17) | 1.00 (0.96–1.11) | 1.00 (0.94–1.07) | 0.97 (0.93–1.04) | < 0.001 | ||
The normal range of liver function indexes: ALB 35–55 g/L, AST 15–40 U/L, ALT 9–50 U/L, TBIL 3.4–17.1 µmol/L,GGT 10–60 U/L,ALP 45–125 U/L,PT 10–13 s,INR 0.5–1.2. The types of liver damage: i) hepatocellular: R ≥ 5; ii) cholestatic type: R ≤ 2; iii) mixed type: 2 < R < 5; iv) abnormal liver biochemical examination: abnormal ALT and ALP but unmet the above criteria. M (IQR): median (interquartile range); n (%): number of cases (percentage)
Among 322 DILI cases, 153 cases (47.5%) were caused by TCM, 141 cases (43.8%) by WMs, 3 cases (0.9%) by health products, and the remaining 25 cases (7.8%) by the combination of Chinese and WMs. The detailed distribution of DILI causative drugs is shown in Figure 2. Except for TCM, the most common causative drugs were anti-tumor drugs (95 cases, 29.5%), which contained traditional chemotherapeutic drugs (e.g., pemetrexed, oxaliplatin and cisplatin, and tegafur), targeting drugs (e.g., gefitinib, icotinib, alectinib, crizotinib, and rituximab), immune regulators (e.g., nivolumab, camrelizumab, tislelizumab, pembrolizumab, and ipilimumab), and endocrine therapeutic drugs (e.g., flutamide).
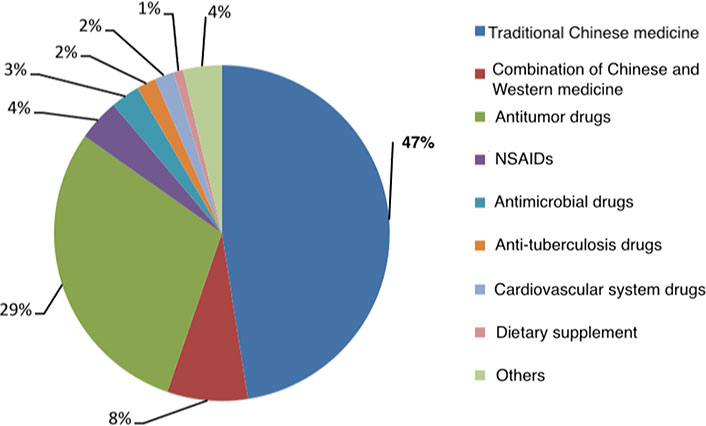
Major categories of causative drugs of DILI. NSAIDs: nonsteriodal anti-inflammatory drugs
Among the 153 cases (47.5%) of liver injury caused by TCM, 127 cases (83.0% of the TCM-induced DILI) were caused by herbal medicine, 25 cases were caused by Chinese patent medicine, of which 60 cases were unknown about the compositions and 2 cases were unknown about the name of Chinese patent medicine. According to the components of TCM with recorded hepatotoxicity on Hepatox website in China (www.hepatox.org), the specific components of top 10 TCMs were listed in Table 2 [16–27].
Top 10 toxic components of TCMs causing DILI
| Herbal medicine | Cases | Hepatotoxic components |
|---|---|---|
| Polygonum multiflorum | 23 | Anthraquinones (emodin, aloe emodin, rhein), gallic acid [16] |
| Pinellia ternata | 12 | Pinellia ternata aqueous extract [17], alkaloids (ephedrine), lectin protein [18] |
| Psoralea corylifolia | 11 | Psoralen and isopsoralen [19] |
| Chinese rhubarb | 11 | Tannin and its components are emodin, aloe emodin, rhein, and emodin methyl ether [20] |
| Salvia miltiorrhiza | 11 | Terpenoids [21] |
| Bupleurum | 8 | Bupleurum water extract, Bupleurum alcohol extract, Bupleurum saponin, and volatile oil components [22] |
| Mentha spicata | 7 | Peppermint volatile oil and its aqueous extract [23] |
| Panax notoginseng | 8 | Pyrrolizidine alkaloids (Senecio base, phenanthroline [24]) |
| Alisma orientalis | 6 | Terpenoids and alkaloids [25] |
| Pueraria lobata | 6 | Santai saponins, alkaloids, and isoflavones [26, 27] |
As shown in Table 3, among 322 DILI patients in this study, there were 247 cases with severity ≤ level 3 assigned in non-DILF group, and 75 cases with severity ≥ level 4 (including 67 cases of level 4 and 8 cases of level 5) assigned in DILF group. Univariate analysis showed that there were significant differences between the DILF and non-DILF groups in clinical classification, comorbidity of gallbladder lesions (including gallstone, gallbladder polyp, gallbladder adenomyosis, and gallbladder cholesterol crystallization) or malignant tumors, categories of causative drugs, initial title of ANA, initial levels of liver function parameters (TBIL, ALT, AST, ALP, GGT, and PT). Higher DILF occurrence was found in the CM group than in the WM group and the combination of CM and WM group. More DILF patients were found in the cholestatic type of liver injury than those in the hepatocellular type and the mixed type, as well as patients of those with ANA positive than negative. However, there is no significant difference between DILF and non-DILF groups in age, sex, latency period, comorbidities as hypertension, diabetes, coronary heart disease, fatty liver, chronic hepatitis B, and malignant tumors. See Table 3 for details.
Single factor analysis for the risk factors of DILF
| Variates | Severity | P value | ||||
|---|---|---|---|---|---|---|
Level ≤ 3 (n = 247; 76.7%) | Level ≥ 4 (n = 75; 23.3%) | |||||
| Gender [n (%)] | ||||||
| Male | 95 (38.5) | 31 (41.3) | 0.655 | |||
| Female | 152 (61.5) | 44 (58.7) | ||||
| Age [M (IQR)] | 54 (42–64) | 59 (50–67) | 0.515 | |||
| Clinical classification | ||||||
| Hepatocellular | 133 (53.8) | 50 (66.7) | < 0.001 | |||
| Cholestatic | 11 (4.5) | 12 (16.0) | ||||
| Mixed | 14 (5.7) | 4 (5.3) | ||||
| Abnormal liver biochemical examination | 89 (36.0) | 9 (12.0) | ||||
| Latency (days) | ||||||
| < 5 | 13 (5.3) | 2 (2.7) | 0.153 | |||
| 5–90 | 197 (79.7) | 55 (73.3) | ||||
| > 90 | 37 (15.0) | 18 (24.0) | ||||
| Comorbidities | ||||||
| Hypertension [n (%)] | Yes | 71 (28.7) | 19 (25.3) | 0.564 | ||
| No | 176 (71.3) | 56 (74.7) | ||||
| Diabetes [n (%)] | Yes | 14 (5.7) | 5 (6.7) | 0.781 | ||
| No | 233 (94.3) | 70 (93.3) | ||||
| Coronary heart disease [n (%)] | Yes | 18 (7.3) | 5 (6.7) | 0.855 | ||
| No | 129 (92.7) | 70 (93.3) | ||||
| Gallbladder lesion [n (%)] | Yes | 64 (25.9) | 47 (62.7) | < 0.001 | ||
| No | 183 (74.1) | 28 (37.3) | ||||
| Fatty liver [n (%)] | Yes | 65 (26.3) | 20 (26.7) | 0.952 | ||
| No | 182 (73.7) | 55 (73.3) | ||||
| Chronic hepatitis B [n (%)] | Yes | 12 (4.9) | 3 (4.0) | 0.757 | ||
| No | 235 (95.1) | 72 (96.0) | ||||
| Malignant tumor [n (%)] | Yes | 112 (45.3) | 10 (13.3) | < 0.001 | ||
| No | 135 (54.7) | 65 (86.7) | ||||
| ANA [n (%)] | Negative | 163 (66.0) | 28 (37.3) | < 0.001 | ||
| 1:100 | 55 (22.3) | 29 (38.7) | ||||
| ≥ 1:320 | 29 (11.7) | 18 (24.0) | ||||
| Categories of causative drugs | ||||||
| Drug categories [n (%)] | CM | 98 (59.7) | 58 (77.3) | < 0.001 | ||
| WM | 131 (53.0) | 10 (13.3) | ||||
| CM + WM | 18 (7.3) | 7 (9.3) | ||||
| Laboratory parameters of liver function (at presentation) [M (IQR)] | ||||||
| TBIL | 16.9 (8.7–53.8) | 183.6 (122.4–259.3) | < 0.001 | |||
| ALT | 240 (78–974) | 898 (330–1,452) | - | |||
| AST | 173 (53–595) | 753 (238–1,106) | - | |||
| ALP | 110 (78–169) | 174 (132–263) | - | |||
| GGT | 104 (47–250) | 193 (116–322) | < 0.001 | |||
| PT | 11.5 (10.8–12.3) | 12.6 (11.6–15.5) | < 0.001 | |||
The variables with P < 0.05 in the above univariate analysis underwent multivariate logistic regression analysis. The results showed that pre-existing gallbladder disease, clinical classification, initial TBIL, initial PT, and initial title of ANA were the independent risk factors for DILF (Table 4). M (IQR): median (interquartile range); n (%): number of cases (percentage); -: none
Multivariate logistic regression analysis for the risk factors of DILF
| Variable | β | SE | Wald | P value | OR value (95% CI) |
|---|---|---|---|---|---|
| TBIL | 0.042 | 0.007 | 39.098 | < 0.001 | 1.043 (1.029–1.057) |
| PT | 0.420 | 0.134 | 9.880 | 0.002 | 1.522 (1.171–1.977) |
| Clinical classification | |||||
| Hepatocellular | - | - | 10.519 | 0.015 | - |
| Cholestatic | 2.096 | 1.329 | 2.487 | 0.115 | 8.132 (0.601–110.021) |
| Mixed | –0.779 | 1.267 | 0.378 | 0.539 | 0.459 (0.038–5.501) |
| Abnormal liver biochemical examination | 2.813 | 1.072 | 6.884 | 0.009 | 16.664 (2.038–136.288) |
| Gallbladder lesion | 1.220 | 0.548 | 4.960 | 0.026 | 3.388 (1.158–9.914) |
| ANA | |||||
| Negative | - | - | 4.600 | 0.100 | - |
| 1:100 | 1.340 | 0.626 | 4.578 | 0.032 | 3.818 (1.119–13.024) |
| ≥ 1:320 | 0.485 | 0.786 | 0.380 | 0.538 | 1.624 (0.348–7.584) |
SE: standard error; -: none
Within the 322 patients that were included in the present study, 236 patients were recovered, 61 patients were chronicity, 11 patients died, and 14 patients were lost of follow-up. The 61 cases were grouped as the chronicity group, and 236 cases were grouped as the recovered group. Univariate analysis was used for analyzing the risk factors of chronic DILI. It was shown that age, clinical types, comorbidities of fatty liver and malignant tumor, drug categories of Chinese medicine and anti-tumor WM, and T0.5ALT, T0.5AST, T0.5GGT were significantly different between the recovered group and the chronicity group (P < 0.05). See Table 5 for details. Further comparison between these two groups shown that the cholestatic type of DILI patients were more prone to chronicity than those of the hepatocellular type (P < 0.001). There was no significant difference in gender, comorbidities, clinical symptoms, ANA title, and categories of causative drugs and the therapeutic drugs (including glucocorticoids) between these two groups.
Univariate analysis of risk factors for chronic DILI
| Variable | Recovered (n = 236) | Chronicity (n = 61) | P value | |
|---|---|---|---|---|
| Gender [n (%)] | Male | 91 (38.6) | 24 (39.3) | 0.911 |
| Female | 145 (61.4) | 37 (60.7) | ||
| Age [M (IQR)] | 55 (43–65) | 59 (51–65) | 0.026 | |
| Clinical classification | Hepatocellular | 156 (66.1) | 15 (24.6) | < 0.001 |
| Cholestatic | 10 (4.2) | 8 (13.1) | ||
| Mixed type | 14 (5.9) | 3 (4.9) | ||
| Abnormal liver biochemical examinations | 56 (23.7) | 35 (57.4) | ||
| Latency (days) | < 5 | 12 (5.1) | 2 (3.3) | 0.731 |
| 5–90 | 185 (78.4) | 47 (77.0) | ||
| > 90 | 39 (16.5) | 12 (19.7) | ||
| Comorbidities [n (%)] | ||||
| Hypertension | Yes | 62 (26.3) | 20 (32.8) | 0.310 |
| No | 174 (73.7) | 41 (67.2) | ||
| Diabetes | Yes | 14 (5.9) | 3 (4.9) | 0.761 |
| No | 222 (94.1) | 58 (95.1) | ||
| Coronary heart disease | Yes | 14 (5.9) | 7 (11.5) | 0.220 |
| No | 222 (94.1) | 54 (88.5) | ||
| Gallbladder lesion | Yes | 84 (35.6) | 16 (26.2) | 0.168 |
| No | 152 (64.4) | 45 (73.8) | ||
| Fatty liver | Yes | 52 (22.0) | 21 (34.4) | 0.045 |
| No | 184 (78.0) | 40 (65.6) | ||
| Chronic hepatitis B | Yes | 13 (5.5) | 1 (1.6) | 0.351 |
| No | 223 (94.5) | 60 (98.4) | ||
| Malignant tumor | Yes | 76 (32.2) | 38 (62.3) | < 0.001 |
| No | 160 (67.8) | 23 (37.7) | ||
| ANA | Negative | 142 (60.2) | 38 (62.3) | 0.503 |
| 1:100 | 65 (27.5) | 13 (21.3) | ||
| ≥ 1:320 | 29 (12.3) | 10 (16.4) | ||
| Drug categories [n (%)] | CM | 121 (51.3) | 19 (31.1) | 0.005 |
| Antimicrobial | 8 (3.4) | 1 (1.6) | 0.770 | |
| NSAIDs | 12 (5.1) | 1 (1.6) | 0.411 | |
| Anti-tumor | 53 (22.5) | 35 (57.4) | < 0.001 | |
| Anti-tuberculosis | 5 (2.1) | 0 (0.0) | 0.587 | |
| Cardiovascular | 5 (2.1) | 1 (1.6) | 0.813 | |
| CM-WM | 21 (8.9) | 3 (4.9) | 0.451 | |
| Others | 11 (4.7) | 1 (1.6) | 0.482 | |
| Therapeutic drugs [n (%)] | Single | 19 (8.1) | 10 (16.4) | 0.094 |
| Two drugs | 42 (17.8) | 13 (21.3) | ||
| Three drugs or more | 175 (74.2) | 38 (62.3) | ||
| Glucocorticoids [n (%)] | Yes | 84 (35.6) | 16 (26.2) | 0.168 |
| No | 152 (64.4) | 45 (73.8) | ||
| T0.5TBIL (day) [M (IQR)] | 6 (0–12) | 0 (0–13) | 0.186 | |
| T0.5ALT (day) [M (IQR)] | 8 (5–14) | 20 (8–62) | < 0.001 | |
| T0.5AST (day) [M (IQR)] | 7 (4–12) | 19 (8–76) | < 0.001 | |
| T0.5ALP (day) [M (IQR)] | 7 (0–15) | 7 (0–30) | 0.288 | |
| T0.5GGT (day) [M (IQR)] | 15 (8–27) | 30 (10–75) | 0.001 | |
M (IQR): median (interquartile range); n (%): number of cases (percentage); T0.5TBIL, T0.5ALT, T0.5AST, T0.5ALP, T0.5GGT: time when TBIL, ALT, AST, ALP, and GGT decrease from peak to below 50%, respectively
The variables with P < 0.05 in univariate analysis were included in the multivariate logistic regression model. The results showed that clinical classification, T0.5GGT, T0.5AST were independent risk factors for chronic DILI (Table 6). ROC curve shows that the area under the curve (AUC), sensitivity, and specificity of the combined prediction model are better than those of single factors (Figure 3). The AUC of the combined prediction model was 0.812 (95% CI: 0.748–0.876, P < 0.001), while the AUC of T0.5AST was 0.748 (95% CI: 0.674–0.822, P < 0.001), with a cut-off value of 8.5 days. The AUC of T0.5GGT was 0.644 (95% CI: 0.551–0.737, P = 0.001), with a cut-off value of 29.5 days. See Table 7 for details.
Multivariate regression analysis of risk factors for chronic DILI
| Variable | β | SE | Wald | P value | OR value (95% CI) |
|---|---|---|---|---|---|
| T0.5ALT (day) | 0.020 | 0.006 | 10.050 | 0.002 | 1.020 (1.008–1.032) |
| T0.5GGT (day) | 0.019 | 0.006 | 9.231 | 0.002 | 1.019 (1.017–1.031) |
| Clinical classification | |||||
| Hepatocellular | - | - | 13.137 | 0.004 | - |
| Cholestatic | 1.875 | 0.565 | 11.107 | 0.001 | 6.520 (2.155–19.728) |
| Mixed | 0.797 | 0.704 | 1.281 | 0.258 | 2.220 (0.558–8.829) |
| Abnormal liver biochemical examinations | 1.069 | 0.425 | 6.335 | 0.012 | 2.911 (1.267–6.690) |
T0.5ALT, T0.5GGT: time when ALT and GGT decrease from peak to below 50%, respectively; -: none
Chronic DILI prediction model, T0.5AST, and T0.5GGT prediction efficiency analysis
| Variable | AUC | P value | Cut-off value | Maximum Jordan index | 95% CI |
|---|---|---|---|---|---|
| Combination | 0.812 | < 0.001 | 0.209 | 0.499 | 0.748–0.876 |
| T0.5AST (day) | 0.748 | < 0.001 | 8.5 | 0.386 | 0.674–0.822 |
| T0.5GGT (day) | 0.644 | 0.001 | 29.5 | 0.345 | 0.522–0.762 |
T0.5AST, T0.5GGT: time when AST and GGT decrease from peak to below 50%, respectively
In recent years, the incidence rate of DILI has gradually increased, which may be related to the clinical application of a large number of new drugs, the increase of clinical diagnosis rate, and the increased usage of Chinese herbal medicine and health care products [28, 29]. Most DILI patients recover after drug withdrawal, but some can progress to DILF, chronicity, or even death. Caution should be taken on high-risk patients of DILF and chronicity so as to provide timely prevention and treatment.
A relatively higher portion of female patients (60.9%) was found in the present DILI study. This was in line with previous reports that female gender was risk factor for drug hepatotoxicity [30]. However, the female gender was not associated with a higher risk of worse outcomes as previously reported by Lucena et al. [31]. The reason for the discrepancy was so far unknown, which warrant further investigation in future studies.
The common clinical type of DILI was hepatocellular type (56.8%) (Table 5). Patients with malignant tumors were prone to develop cholestatic liver injury. This may be associated with the complex medication they received and their generally severe condition. In addition, a higher portion of patients with cholestatic type of DILI was found to progress to chronicity than those with hepatocellular type of DILI, and the portion of DILF in the cholestatic type of DILI patients was higher than that those in hepatocellular type and mixed type.
The most frequent initial symptoms of DILI were gastrointestinal manifestation (such as abdominal discomfort, anorexia, nausea, and vomiting), jaundice, choluria, and dizziness/fatigue, timely liver function examination and medical treatment should be carried out during medical practice.
It was revealed that TCMs were the most common causative drugs of DILI, and most cases in the present study were herbal-induced liver injury (HILI). Many Chinese herbal medicines have been used as adjuvant therapies to prevent and treat diseases or promote health all over the world. In the past, there was misintelligence that TCMs were all safe. However, the global ALF research group reported that 20–40% ALF in DILI’s was due to the usage of herbal and dietary supplements [32]. Herbal medicine is also the main cause of DILI as reported by South Korea and Singapore [33, 34]. In China, Chinese herbal medicine, herbal dietary products, and anti-tuberculosis drugs are the main causative drugs leading to the occurrence of the disease [9]. In the present study, TCMs account for 47.5% of causative drugs of DILI and 83.0% of these cases were HILI. The composition and formula of TCMs are complicated with known and unknown adverse reactions. It is very necessary to analyze TCM and HILI and inquisite the toxic ingredients.
In this study, it was found that the common components of TCM taken by DILI patients were Polygonum multiflorum, Pinellia ternata, Psoralea corylifolia, Chinese rhubarb, Salvia miltiorrhiza, Bupleurum, Mantha, Panax notoginseng, Alisma orientalis, and Pueraria, which had been reported to induce liver toxicity in Chinese medicinal literature (Table 2). As WM, DILI caused by TCM is mostly heterogeneous. The happen of TCM-induced DILI may be associated with dosage of medication, course of treatment, individual genetic background, and other susceptible factors. In addition, the clinical application of TCM often involves a combination of multiple herbs or components, rather than a single component. Toxicological analysis is required to clarify the toxic components and compatibility of TCM causing liver injury so as to provide clinical reference and control strategy. Common toxic components of TCM include alkaloids, glycosides, diterpenoids and lactones, anthraquinones, toxic proteins, and heavy metals. It should be suggested that patients with risk factors carefully use the above TCM ingredients and closely monitor liver function.
It is notably that 8 cases of DILI were caused by Panax notoginseng, which has been reported to induced hepatic venule occlusion syndrome with serious liver injury [24, 35]. Its toxic component has been identified as double thick pyrrolidine alkaloid [24]. There are essential differences between Gynura segetum Merr. (Tusanqi) and Sedum aizoon L. (non-toxic Panax notoginseng). Awareness should be raised with strengthen publicity to reduce the misuse of Tusanqi by people.
At present, there are few reports on liver injury caused by Pinellia ternata. Pinellia ternata is a dry tuber of Araceae plants. Pinellia ternata mainly prescribed for the treatment of diseases in human digestive system, endocrine system, cardiovascular system, and vomiting after chemotherapy. Pinellia ternata decoction is effective in treating functional dyspepsia and all types of chronic gastritis. It may have function in promoting gastrointestinal motor activity, anti-helicobacter pylori, immune regulation, and protecting gastric mucosa [36]. In addition, prescriptions such as Banxia Houpu decoction, Banxia Kucao decoction, and Banxia Xiexin decoction have been used for the treatment of hepatitis and early liver cirrhosis [37]. However, it has also been reported that Pinellia ternata may induce hepatotoxicity. Pretreatment with 59.6 g/kg Pinellia ternata significantly increased the levels of serum ALT and AST [38]. Guo et al. [39] showed that low-dose Pinellia ternata slightly reduced liver injury caused by acetaminophen, whereas high-dose Pinellia ternata aggravated the liver injury. Zhang et al. [40] found that a single administration of the water extract of Pinellia ternata in mice induce hepatotoxic injury in a time and dose dependent manner. Wu et al. [41] found that Pinellia ternata significantly inhibited the activity of cytochrome P450 isoenzyme 3A (CYP3A) in rats. CYP3A is the most abundant P450 enzyme in the liver and participates in the metabolism of almost 40–50% drugs [42]. The potential hepatotoxicity induced by Pinellia ternata warrants more mechanism investigation.
The common DILI causative Western drugs in this study were antitumor drugs, NSAIDs, and antibiotics. Antitumor drugs had been reported to be the second largest cause of DILI, accounting for about 20–25% of DILI [43]. In addition to the chemotherapeutic drugs that often lead to liver injury in the past, tyrosine kinase inhibitors (TKIs, such as gefitinib, Ektinib) and immune checkpoint inhibitors (ICIs), which are emerging and increasingly used in past decade to achieve certain efficacy in the treatment of malignant tumors, may cause liver injury as one of their major adverse reactions to dampen their usages. It has been reported that 18.5% of gefitinib treated patients appeared liver injury [44]. The mechanism of TKI-induced DILI may be related to a decrease in the activity of drug metabolic enzymes such as CYP3A4 [45], abnormal expression of endoplasmic reticulum stress pathway and apoptosis related genes [46]. It has also been reported that the active metabolites of icotinib by CYP2C19 polymorphism may cause hepatocyte injury [47]. In addition, TKIs as traditional chemotherapy drugs and immunosuppressants such as corticosteroid may cause hepatitis B viral reactivation.
Similarly, in recent years, ICIs (such as navulizumab, carrelizumab, pabolizumab, tirelizumab) have made breakthrough in tumor treatment. Its three main targets are cytotoxic T lymphocyte associated antigen-4 (CTLA-4), programmed cell death-1 (PD-1), and its ligand (PD-L1) [48–50]. ICIs can cause immune system imbalance by enhancing anti-tumor immune function, which may lead to T cells attacking normal cells in the body, causing inflammatory state simulating a series of autoimmune diseases, leading to immune-related adverse events (IRAEs). IRAEs affect skin, gastrointestinal tract, endocrine organs, lung, and liver [51]. Previous studies have reported that about 10% of patients treated with ICIs (such as Ipimab) are prone to DILI [52]. Immune mediated DILI is generally not too serious, but it can also lead to liver failure [53], and the typical onset time is 6–14 weeks after the start of treatment [54]. Clinical studies show that the incidence of liver injury in patients treated with single drug is less than 5–10%, and about 1–2% of patients with grade 3/4 liver injury. It is reported that when treated with 3 mg/kg of ipilimumab combined with 1 mg/kg of navumab, about 20–30% of patients observed the increase of grade 3/4 ALT [55], and the incidence of hepatotoxicity increased significantly in patients treated with CTLA-4 and PD-1 [56, 57]. In addition, recent studies have shown that non-alcoholic fatty liver disease (NAFLD) may be a potential risk factor for PD-1 inhibitor related DILI [58]. Drug withdrawal means that the tumor loses drug control and treatment, so further research is needed to help early identification and formulate the best prevention and treatment strategy.
Hy’s rule has been used to predict drug-induced severe liver injury but with limited efficiency. This rule points out that about 10% of patients with hepatocellular DILI with jaundice can progress to DILF or death. In the United States and Sweden, about 13–15% of DILI patients progress to DILF [2, 4]. The present study reported that 75 cases of inpatients (23.3%) have DILF, and the pre-existing gallbladder disease, categories of causative drugs, initial ALP, and initial PT were independent risk factors for DILF (Tables 3 and 4).
Previous studies have shown that gallbladder lesions were related to insulin resistance and diseases including type 2 diabetes, obesity, NAFLD, and atherosclerosis [59–61]. Gallbladder lesions may induce or aggravate insulin resistance in susceptible individuals [62, 63], alter the bile acid metabolism [bile acids can activate specific bile acid receptors, such as farnesol X receptor and Takeda G protein-coupled receptor 5 (TGR5), so as to participate in the mediation of glucose and lipid metabolism]. Patients with gallbladder lesions should pay special attention when taking drugs that may cause hepatotoxicity.
Our study found that the higher the initial TBIL, the more likely DILF occurred, which was the same with some previous studies. In addition, the prolongation of the initial PT value is also a relevant factor of DILF. TBIL and PT reflect the excretion and synthesis function of the liver. Attention should be paid to patients whose above indexes are significantly increased at presentation.
It was found in the present study that 26 patients (11.6%) had chronic DILI. Pang et al. [64] analyzed 57 cases (10%) of 570 cases of chronic DILI and pointed out that chronic DILI is more common in cholestatic patients. We also found more patients with cholestatic progressed to chronic liver injury (Table 6). In addition, T0.5GGT, T0.5ALT, and T0.5AST were significantly prolonged in patients with chronicity. A further multivariate logistic regression analysis showed that T0.5AST and T0.5GGT were independent risk factors for chronic DILI, which may have certain predictive values for chronic DILI, with cut-off values of 8.5 days and 29.5 days, respectively. This increment in T0.5 for both parameters in chronic DILI can be associated with different causes. For example, severe liver injured cases usually develop renal dysfunction, and AST activity is extremely high in the liver but also kidneys [65]. Given the fact that liver injury severe cases usually develop renal dysfunction, severe cases usually tend to be the most prolonged. More studies are needed to verify the predictive value of liver biochemical tests for chronic DILI.
The present study had several limitations. First, it was retrospective and from a single institution, which may introduce the patient selection bias. Second, all the study cases were recruited from a hospitalization registry, thus it would be expected to have a higher proportion of severe and chronic cases. Despite these limitations, we believe that the present study is crucial for better understanding and improving the management of severe and chronic DILI in China. Prospective and multicenter studies will be required to validate the findings in present study in the future.
Caution should be taken for DILI in the usage of clinically prescribed and non-prescription drugs including herbal medicine. TCM is the most common cause of DILI. Anti-tumor drugs have become the second leading cause of DILI. Both TKIs and ICIs can cause DILI and affect the treatment of the primary diseases. The combination of gallbladder disease, clinical classification, initial TBIL, initial PT, and initial title of ANA are independent risk factors for DILF. Clinical classification, T0.5AST (8.5 days) and T0.5GGT (29.5 days) are independent risk factors to predict chronic DILI. The patients should be closely monitored and provided with prevention and treatment strategies.
ALB: albumin
ALF: acute liver failure
ALP: alkaline phosphatase
ALT: alanine aminotransferase
ANA: antinuclear antibodies
AST: aspartate aminotransferase
AUC: area under the curve
CI: confidence interval
CM: traditional Chinese medicine and health care product
CMV: cytomegalovirus
CYP3A: cytochrome P450 isoenzyme 3A
DILF: drug-induced liver failure
DILI: drug-induced liver injury
EBV: Epstein-Barr virus
GGT: γ-glutamyltransferase
HILI: herbal-induced liver injury
ICIs: immune checkpoint inhibitors
INR: international normalized ratio
NSAIDs: nonsteriodal anti-inflammatory drugs
ORs: odds ratios
PD-1: programmed cell death-1
PT: prothrombin time
ROC: receiver operating curve
RUCAM: Roussel Uclaf Causality Assessment Method
TBIL: total bilirubin
TCMs: traditional Chinese medicines
TKIs: tyrosine kinase inhibitors
ULN: upper limit of normal
WMs: Western medicines
QW: Data curation, Investigation, Methodology, and Writing—original draft. LL: Methodology and Writing—review & editing. XZ and JY: Data curation, Writing—original draft, and Writing—review & editing. JG: Methodology, Writing—original draft and Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The study was approved by the ethical committee of Zhongshan Hospital affiliated to Fudan University, Shanghai, China. The study protocol conformed to the provisions of the Declaration of Helsinki.
Informed consent to participate in the study was obtained from all participants.
Informed consent to publication was obtained from relevant participants.
All the citations and data included in this manuscript are available upon request by contact with the corresponding author.
This work was funded by the National Fund of Nature Science of P.R. China [91129705, 81070340] and Shanghai Pujiang Talent Program [09PJ1402600]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Gulcin Cakan-Akdogan ... Ozlen Konu
Alejandro Cueto-Sánchez ... Marina Villanueva-Paz
Hanghang Wu ... Francisco Javier Cubero
Miren García-Cortés ... Alberto García-García
