Affiliation:
1Department of Cell Biology, Radboud Institute for Molecular Life Sciences, Radboud University Medical Center, 6525 GA, Nijmegen, The Netherlands
2Vision Medical Communications, 6525 JL, Nijmegen, The Netherlands
Affiliation:
1Department of Cell Biology, Radboud Institute for Molecular Life Sciences, Radboud University Medical Center, 6525 GA, Nijmegen, The Netherlands
Email: alessandra.cambi@radboudumc.nl
ORCID: https://orcid.org/0000-0003-1597-1582
Explor Med. 2021;2:167–185 DOI: https://doi.org/10.37349/emed.2021.00040
Received: February 03, 2021 Accepted: March 28, 2021 Published: April 30, 2021
Academic Editor: Yang Shi, RWTH Aachen University Clinic, Germany
The article belongs to the special issue Nanomedicine and Cancer Immunotherapy
The limitations of current cancer treatments have stimulated the application of nanotechnology to develop more effective and safer cancer therapies. Remarkable progress has been made in the development of nanomedicine to overcome issues associated with conventional cancer treatment, including low drug solubility, insufficient targeting, and drug resistance. The modulation of nanoparticles allows the improvement of drug pharmacokinetics, leading to improved targeting and reduced side effects. In addition, nanoparticles can be conjugated to ligands that specifically target cancer cells. Furthermore, strategies that exploit tumor characteristics to locally trigger drug release have shown to increase targeted drug delivery. However, although some clinical successes have been achieved, most nanomedicines fail to reach the clinic. Factors that hinder clinical translation vary from the complexity of design, incomplete understanding of biological mechanisms, and high demands during the manufacturing process. Clinical translation might be improved by combining knowledge from different disciplines such as cell biology, chemistry, and tumor pathophysiology. An increased understanding on how nanoparticle modifications affect biological systems is pivotal to improve design, eventually aiding development of more effective nanomedicines. This review summarizes the key successes that have been made in nanomedicine, including improved drug delivery and release by polymeric nanoparticles as well as the introduction of strategies that overcome drug resistance. In addition, the application of nanomedicine in immunotherapy is discussed, and several remaining challenges addressed.
Despite remarkable progress in our understanding of the biological background of cancer and the continuous improvement of cancer therapies, cancer remains one of the leading causes of death. Conventional therapies have improved patient survival but suffer from several limitations. For instance, anti-cancer drugs frequently fail because of their poor tumor selectivity and high toxicity in healthy tissue. Even when the treatment is initially effective, development of drug resistance is a common threat for long-term survival and cure [1, 2].
Nanomedicine is a novel and rapidly evolving field that might overcome many of the limitations of current anti-cancer drugs. Its nanosize, material and ability for surface modification provide unique features that can dramatically improve pharmacokinetics of incorporated therapeutic agents. Modifications can improve solubility, prolong circulation time, avoid clearance by the immune system or allow for controlled drug release. The nanosize allows drug accumulation in tumor tissue by passive targeting, which relies on the enhanced permeability and retention (EPR) effect. Active targeting on the other hand, comprises functionalized nanoparticles that specifically target molecules expressed on cancer cells, as this specific targeting has shown promise to increase the drug concentration at the site of action while limiting side effects.
The possibility for specific targeting also lends itself to individualized therapies. Tumor heterogeneity between patients is one of the main challenges in the development of effective drugs, and has prompted the emergence of personalized cancer medicine. Recent advances in nanomedicine have the potential to develop into effective and personalized anti-cancer therapies.
Over the past years, various nanoparticles have been developed for drug delivery to the tumor, including viruses, lipid based and polymeric nanoparticles [3]. This has led to a large variation in shape (e.g., spherical or rod-like), size, surface coating and material (e.g., lipid layer or polymer aggregate). These physicochemical properties can influence pharmacokinetic and biological parameters such as functionalization, toxicity, drug release, etc. (Table 1). Several modifications of nanoparticle physicochemical properties aimed at improving pharmacokinetics and targeting, or at reducing immunogenicity and toxicity have been reported (reviewed in [4, 5]).
Nanoparticle physicochemical properties influence pharmacokinetic and biological parameters
| Shape | Size | Surface | Material |
|---|---|---|---|
| Functionalization | Toxicity | Toxicity | Toxicity |
| Toxicity | Biodistribution | Biodistribution | Biodistribution |
| Stability | Opsonization | Stability | Opsonization |
| Biodistribution | Cellular uptake | Opsonization | Cellular uptake |
| Opsonization | Clearance | Tissue retention | Drug release |
| Cellular uptake | EPR effect | Clearance | |
| Tissue retention | Tissue retention | Tissue retention |
The possibility of modifying nanoparticle properties to circumvent limitations of current cancer drugs stimulated rapid progress to clinical use. Several therapeutic nanoparticles are approved for clinical use (Table 2) or are currently investigated in clinical trials. Most clinically approved nanomedicines consist of liposomes as drug carriers including Doxil, DaunoXome, and the more recent VYXEOS (Table 2). Other types of nanoplatforms that are approved for clinical use involve chemotherapeutics bound to proteins and drug-loaded polymers such as Eligard, which has been used to treat prostate cancer since 2002. Although most approved nanomedicine rely on passive targeting, targeted drug delivery gained attention to increase drug specificity. For instance, the antibody-drug conjugate Polivy has recently been approved to treat relapsed diffuse large B-cell lymphoma. Nevertheless, new nanoparticle platforms and approaches are emerging rapidly. Promising results have been obtained with new strategies such as small interfering RNA (siRNA) delivery to silence genes, targeted photoimmunotherapy (PIT), and synthetic dendritic cells to induce immune responses [6–8].
Examples of clinically approved nanomedicine
| Year of approval | Name | Nanotechnology platform | Therapeutic agent | Indication | Refs |
|---|---|---|---|---|---|
| 1995 (FDA)1996 (EMA) | Doxil/Caelyx | Liposome (PEGylated) | Doxorubicin | HIV-associated Kaposi sarcoma, ovarian cancer,metastatic breast cancer, multiple myeloma | [9] |
| 1996 (FDA) | DaunoXome | Liposome (non-PEGylated | Daunorubicin | HIV-associated Kaposi sarcoma | [10] |
| 2002 (FDA) | Eligard | poly(D, L-Lactide-co-glycolide) (PLGH) | Leuprorelin acetate | Prostate cancer | [11] |
| 2005 (FDA)2008 (EMA) | Abraxane | Nanoparticle albumin bound | Paclitaxel | Advanced non-small-cell lung cancer, metastatic breast cancer, metastatic pancreatic cancer | [12] |
| 2006 (FDA) | Oncaspar | polyethyleneglycol (PEG) protein conjugate | L-asparaginase | Leukemia | [13] |
| 2009 (EMA) | MEPACT | Liposome (non-PEGylated) | Mifamurtide | Osteosarcoma | [14] |
| 2015 (FDA) | MM-398/Onivyde | Liposome (PEGylated) | Irinotecan | Metastatic pancreatic cancer | [15] |
| 2017 (FDA) | VYXEOS | Liposome | Daunorubicin and cytarabine | Acute myeloid leukemia | [16] |
| 2019 (FDA) | Polivy (polatuzumab vedotin-piiq) | Antibody-drug conjugate | Monomethyl auristatin E | Relapsed diffuse large B-cell lymphoma | [17] |
FDA: Food and Drug Administration; EMA: European Medicines Agency
Nanomedicine might seem the most appropriate approach in the fight against cancer, and could possibly even lead to the cure in the future. Yet, many challenges lie ahead, ranging from difficulties in design to limitations in clinical translation. And although promising, only a limited number of nanotechnology-based therapies has so far reached the clinic and still many nanomedicines fail to show benefits compared to conventional treatments [18, 19]. Often this is due to lack of thorough experimental approaches that should evaluate the effects of nanomedicine on basic biological processes in cells and tissues. This review highlights recent advances that exploit unique promising features of polymeric nanoparticles and elaborates on remaining challenges (Figure 1).
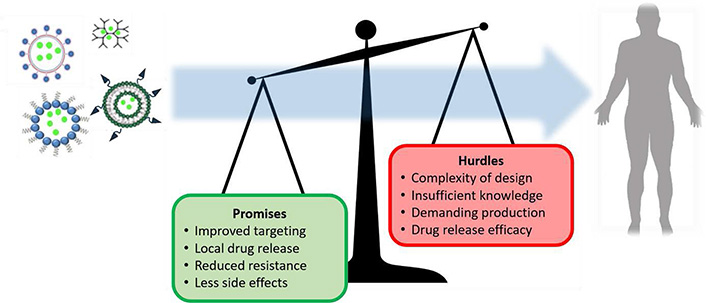
Schematic summary of promising aspects and remaining challenges of nanomedicine in cancer therapy
Liposomes were the first nanoparticles that were approved for therapeutic use in the clinic. They have been used to increase the circulation time and specific uptake of the cytostatic drug doxorubicin [20, 21]. Free doxorubicin is an effective chemotherapeutic for several cancer types. However, the severe side effects due to cardiotoxicity limits the administered dose, resulting in low therapeutic efficacy [22]. Incorporation of doxorubicin in liposomes enhances uptake in tumor cells and results in a more gradual drug release, thereby preventing cardiotoxic effects [11, 23–25]. Nevertheless, liposomal doxorubicin has failed to increase overall survival [26–28], which is likely attributed to the inefficient cargo release due to lack of triggered release after entering tumor tissue. This emphasizes the need for controlled release systems such as biodegradable drug carriers or modifications that allow drug release upon triggers from the tumor environment.
Nanoparticles have also attracted widespread interest as delivery vehicles for nucleic acids. siRNA is widely used to silence gene expression, but therapeutic application of free siRNA is limited due to its low stability in vivo [29]. Cationic polymers such as lipoplexes and polyplexes are commonly used as delivery vehicles, and are taken up by endocytotic pathways [30]. Encapsulation of siRNA in these nanoparticles increases stability, permeability, and cellular uptake [29, 31, 32]. Therefore, many studies aimed to investigate the effects and mechanisms of siRNA nanoparticle delivery. Internalization of siRNA can occur through different mechanisms that are dependent on many properties of the polymeric vehicle, such as charge, size and conjugation of cell penetrating peptides, antibodies, or aptamers [33].
Knowledge on the mechanism of nanoparticle uptake is critical to aid development of siRNA release systems. The majority of nanoparticles are taken up by endocytosis due to their ability to interact with the cell membrane through properties such as receptor targeting ligands or a positive charge. The rapid acidification of endolysosomes causes siRNA degradation, and thus necessitates early endosomal release of siRNA. This has led to the development of nanoparticles functionalized with amines that are protonated at acidic pH, causing osmotic swelling and endosomal lysis [33]. This is also known as the proton sponge effect and although widely accepted, a small endosomal size and low membrane leakiness may be pivotal for sufficient build-up of osmotic pressure to allow proton sponge-based rupture [34]. Based on the comparison of four distinct cell types, endosomal rupture occurred less frequently in cells with smaller endosomes. Moreover, exposure to high polyplex concentrations increased endosomal leakiness, preventing endosomal rupture. In addition, Rejman et al. [35] reported caveolae-mediated endocytosis of polyplexes that may not involve endosomal acidification, indicating other release mechanisms than the proton sponge effect. Caveolae-mediated endocytosis was shown to be the main route for larger particles (> 200 nm), whereas smaller particles were exclusively internalized by clathrin-mediated endocytosis [35]. Alternative to the proton sponge effect, endosomal siRNA escape has been proposed to occur through pore formation by using endosomolytic peptides. Although studies report increased siRNA endosomal escape after incorporation of amines or endosomolytic peptides [36, 37], internalization pathways and siRNA release mechanisms have been poorly addressed.
Live cell imaging has confirmed the association between cytosolic siRNA release and knockdown of targeted genes [38]. However, the release efficiency of nucleic acids from endosomes is considerably low with only a limited number of particles that release siRNA [30]. In addition, both lipoplexes and polyplexes do not rupture completely. Lipoplexes exhibited a more gradual release of siRNA in the cytosol, which could indicate endosomal release through pore formation. Nevertheless, siRNA nanoparticles were able to silence cancer associated genes in several tumor mouse models, leading to a significant decrease in tumor growth [39, 40]. siRNA treatment has also particularly attracted interest to treat triple-negative breast cancer (TNBC) that lack molecular features. Conventional drugs are ineffective because of the inability to target and the highly aggressive nature of TNBC. siRNA nanoparticles were able to silence different oncogenic genes in TNBC mice models [41].
Since escape from the endo-lysosomal system is a rate-limiting step for efficient drug delivery, modification of nanoparticles to bypass this system has gained interest [42, 43]. As described above, nanoparticle size has important implications for uptake mechanisms and could be used to direct uptake to caveolae-mediated endocytosis and thereby prevent cargo degradation in lysosomes. Hence, it could improve nanoparticle cargo release [35, 42]. Other strategies to mediate uptake by the caveolae pathway consist of modifying surface characteristics and shape [42]. Xin. et al. [44] developed nanoparticles that were able to bypass the endo-lysomal pathway by coating rod-like pure drug nanoparticles (PNPs) with hyaluronic acid (HA). These HAPNPs were loaded with caspase 3 to assess protein delivery. The nanoparticles were able to enter cancer cells through binding to the CD44 receptor and uptake by the caveolar pathway. Levels of functional caspase-3 increased by 7-fold in human epithelial colorectal adenocarcinoma cell line, indicating efficient release. HA coating increased tumor accumulation in vivo, but the caveolae-mediated endocytosis was attributed to the rod-like shape [44, 45].
A major problem in conventional cancer therapies is the development of drug resistance. Nanoparticles can prevent drug resistance by delivery of multiple drugs or siRNAs. Delivery of different siRNAs has potential to silence multiple genes simultaneously, including drug resistance genes. Nanoparticles that targeted the epidermal growth factor receptor (EGFR) on cancer cells, were successfully used for co-delivery of doxorubicin and siRNA to silence the drug resistance gene Bcl-2. After co-delivery, lung tumors in mice were more than twice smaller than those treated with doxorubicin or siRNA alone, indicating more effective suppression [6].
Multidrug resistance (MDR) is often caused by increased drug efflux or reduced uptake as a consequence of overexpressed transmembrane transporters. Aptamers, small single stranded DNA sequences, have shown potential to circumvent this resistance mechanism in cancer cells [46]. Liu et al. [47] developed a self-assembling DNA based nanostructure in which an aptamer was used to target nucleolin, a protein that is often upregulated in cancer cells. This was effectively used to deliver and retain doxorubicin in drug-resistant MCF-7 breast cancer cells. In vivo results demonstrated a tumor inhibition by 88% compared to an inhibition rate of 22% after administration of free doxorubicin [48]. The increased efficacy of nanomedicine in MDR is suggested to be a consequence of its different uptake mechanism. Unlike free drugs, targeted nanoparticles are taken up by endocytosis, which improves cellular uptake and circumvents drug efflux transporters.
Besides acquired MDR through increased drug efflux, intrinsic mechanisms including altered apoptosis signalling, play a major role in MDR [49]. Mitochondria have long been hypothesised to be involved in intrinsic MDR because of their role in energy metabolism and apoptosis. Studies have confirmed that an increase in oxidative phosphorylation (OXPHOS) in mitochondria is an important driver for chemoresistance [50–52]. This has stimulated the development of mitochondrial targeting nanoparticles to promote cell apoptosis by selective drug accumulation. Jiang et al. [53] generated paclitaxel containing liposomes that could successfully target cancer cells by the incorporation of 2, 3-dimethylmaleic anhydride (DMA), an amide bond that is cleaved in the acidic tumor microenvironment. Cleavage of DMA reverses nanoparticle charge, which promotes cellular uptake. Mitochondrial uptake was facilitated by the incorporation of the mitochondrial targeting peptide lysine-leucine-alanine and subsequently caused apoptosis in MDR lung cancer cells and in MDR lung cancer cell xenografted mice.
A recent study also demonstrated improved therapeutic outcomes in MDR tumors after mitochondrial drug accumulation [54]. Dendrigraft poly-L-lysine (DGL) nanoparticles, devised as carriers for doxorubicin, contained aptamers to target nucleolin on tumor cells and cytochrome c in mitochondria. Another aptamer allowed drug release that was triggered by the high concentrations of ATP in mitochondria. Mitochondrial drug release induced apoptosis in vitro and in vivo, indicating successful circumvention of acquired and intrinsic MDR by DGL nanoparticles.
Another mechanism of MDR involves the regulation of ceramide content in the cell membrane [55]. Ceramides are membrane lipids and regulate several cellular signalling pathways, including cell differentiation, proliferation, and programmed cell death. Overexpression of the enzyme glucosylceramide synthase, responsible for the conversion of the proapoptotic mediator ceramide to a nonfunctional glycosylceramide, is associated with MDR [55]. Co-administration of ceramide with the chemotherapeutic drug paclitaxel restored apoptotic signalling in MDR human ovarian cancer cells [56]. Moreover, inhibition of glucosylceramide synthase by the drug tamoxifen augmented the cytotoxic effects of paclitaxel in MDR human ovarian adenocarcinoma cells (by 10-fold) and non-MDR ovarian cancer cells (by more than 3-fold) [57]. This finding was confirmed by the increased anti-tumor efficiency after the addition of tamoxifen to paclitaxel-loaded nanoparticles in a xenograft mouse model. So in addition to specific targeting to circumvent MDR, the ability to co-deliver compounds to restore altered signalling pathways is a promising feature of nanomedicine to overcome MDR [57]. Knowledge about the particular MDR mechanism in patients is therefore crucial to decide the best nanoparticle design for the treatment of chemo-resistant tumors.
When nanoparticles are taken up in the cell, they need to release the drug to evoke therapeutic effects. The design of effective release strategies is one of the main challenges encountered in nanomedicine. Endocytosis is the major route for nanoparticle internalization but cargo release is known to be a rate-limiting step and necessitated the need for controlled drug release [58]. Specific characteristics of the tumor microenvironment and endosomes, including the acidic pH and metabolic products, have been exploited to trigger drug release. Furthermore, nanoparticles that release their cargo upon external stimuli such as exposure to ultrasound, light, temperature, or magnetic fields have shown promising as controlled drug release systems [59–64].
Biodegradable polymers allow sustained drug release. Both natural and synthetic polymers are used as drug release systems, but synthetic polymers offer the advantage of a more gradual and controlled release. Polymer degradation causes the release of encapsulated drugs and can be controlled by altering the composition and molecular weight [65, 66]. Polylactides (PLA) and poly (D, L-lactide-co-glycolide) (PLGA) are the most extensively studied non-toxic polymers for controlled drug release [67]. These particles can be easily altered to control the rate of hydrolysis and thereby allow sustained drug release over a period of days to several weeks. Although highly suitable for drug release, PLA and PLGA nanoparticles are not biocompatible with all drugs [68]. Other biodegradable materials are therefore investigated to cover the wide range of available drugs. Polymers such as modified poly(glycerol-adipate) (PGAS), PEGylated poly(ε-caprolactone) (PEG-PCL) or calcium-based biomaterials are promising materials for drug release systems because of their high tunability and low toxicity [69–72].
For localized drug release in cancer cells, several nanoparticles have been designed to release their cargo upon tumor specific stimuli [61]. Cyclodextrin-based nanoplatforms have various biomedical applications for cancer treatment and theranostics because different functionalities can be incorporated to make them responsive to stimuli, such as pH, temperature, redox, enzymes, light and magnetic fields [73]. In addition, polymers and liposomes can be functionalized with groups that alter particle charge after environmental change in pH, causing disruption and subsequent drug release. A recent study demonstrated selective release in tumor cells, triggered by the acidic lysosomal pH and high levels of glutathione in cervical cancer cells [74]. Rhodamine B-modified chitosan was used as a model for macromolecular drugs and aminated fluorescein served as a model for small molecule drugs. Both substances were incorporated into biodegradable organosilica-based core-shell structured nanoparticles, and were only released after uptake in tumor cells. This indicates its potential as a controlled dual drug delivery system. However, in vivo studies are needed to confirm the efficacy and specificity of this drug release system.
Polymers and liposomes have also been used as building blocks for nanoparticles that release their cargo upon exposure to endogenous enzymes [75]. Furthermore, the use of glucose oxidase has attracted increasing interest due to its potential to enhance conventional nanomedicine strategies [75]. Glucose oxidase efficiently catalyzes the oxidization of glucose into hydrogen peroxide and gluconic acid, which can trigger pH-responsive drug release by increasing the acidity of the tumor microenvironment. Moreover, hydrogen peroxide can be converted into toxic hydroxyl radicals that may enhance the toxicity of PIT [76]. Because particles need different characteristics to accumulate in the tissue and internalize in cells, several studies explored hierarchical targeting approaches that exploit changeable particle sizes, switchable charge and activatable surface ligand [77, 78]. These nanoparticles are generally not activated during blood circulation to facilitate passive tumor accumulation, and are then reactivated by external or internal stimuli to stimulate cellular uptake.
As examples of exogenous stimuli applied to control drug release, photosensitive and magnetic release systems are widely investigated because of their convenience to trigger localized release by exposure of light or a magnetic field [62, 79, 80]. Near-infrared (NIR) light provides advantages for its higher tissue penetration as compared to light in the visible spectrum. NIR light was able to trigger release of doxorubicin from poly (ether amine) nanoparticles that contained the dye cyanine [59]. 31.8% of doxorubicin was released after the first four minutes of NIR light exposure and release increased to almost 60% after three cycles of repeated irradiation with one exposure of four minutes every hour, whereas almost no release was seen when NIR light exposure was stopped. In mice with hepatocellular carcinoma, excitation of cyanine was followed by effective tumor cytotoxicity, which was attributed to both photothermal effects and the thermoresponsive release of doxorubicin.
Recent advances show the synergistic effect of combining chemotherapy, photodynamic therapy (PDT) and hyperthermia therapy [81]. Superior cytotoxic effects in vitro were observed after triggering magnetic liposomes that contained the photosensitizer meta-tetra(hydroxyphenyl)chlorin (mTHPC) and the drug doxorubicin [60]. Exposure of a magnetic field induced magnetic hyperthermia that also triggered heat sensitive liposomal doxorubicin release. Additional light exposure to excite m-THPC, almost completely eliminated tumor cells after 12 h of combined therapy. One study that also shows the potential in vivo was executed by Li et al. [82], who designed a human epidermal growth factor receptor 2-targeting liposome with a modified photosensitizer (ICG-ODA). After 5 min of NIR light exposure, ICG-ODA could generate sufficient reactive oxygen species for PDT and triggered release of incorporated doxorubicin in vitro. A maximum of 80% of doxorubicin was released in 4 h after irradiation, but was highly dependent on the ratio of ICG-ODA and total lipid. Mice treated with this dual therapy had an enhanced anti-tumor effect when compared to PDT or doxorubicin treatment alone, indicating both effective PDT and drug release in vivo. Also ultrasound irradiation has shown to enhance the uptake and anti-tumor activity of human serum albumin-bound paclitaxel loaded with the photosensitizer sinoporphyrin sodium [83]. Combining these three modalities was associated with near total arrest of tumor growth inhibition in a tumor mouse model as compared to controls without ultrasound irradiation.
One of the main therapeutic approaches in cancer is to provoke anti-tumor responses by stimulating the immune response. Clinical success of immunotherapy has been achieved for many cancer types, but the highly heterogeneous responses have attracted interest to image immune cell behavior [84]. An important application of nanoparticles is therefore to visualize distribution and monitor efficacy of cell based immunotherapies, for example by loading immune cells with imaging particles such as iron oxide or perfluorocarbon agents for magnetic resonance imaging (MRI) [85]. Imaging particles have provided valuable insights about the localization and interaction of tumor infiltrating immune cells, leading to improvements in immunotherapy. Although different therapeutic approaches have led to great progress, current immunotherapies are limited by their high costs, heterogeneous responses and lack of control. These limitations have recently stimulated the design of nano-sized artificial immune cells, which will be discussed in the next paragraph. Another promising approach for controlled modulation of the immune system is local delivery of immunotherapeutic agents or immune cells by biomaterial scaffold-based drug delivery systems, such as implanted scaffold and injected hydrogels [86]. Other applications of therapeutic nanoparticles in immunotherapy are reviewed by Jia et al. [87].
Recent advances in the design of artificial antigen-presenting cells (aAPCs) are promising to stimulate anti-tumor responses and can overcome many of the limitations of current immunotherapies [8, 88]. Vaccination of natural dendritic cells (DCs) is commonly used to stimulate the immune response but lead to different responses between patients. These heterogenous responses are likely the result of differences in DC signalling and molecular expression. Synthetic variants on the other hand, have opportunities for full control over T cell activation and overcome the need to produce a customized vaccine for each patient. Synthetic DCs could therefore reduce costs by providing off-the-shell therapy in the future.
The design of aAPCs is inspired by natural dendritic cells and can stimulate T cells by incorporation of the required signals: signal 1 to activate T cell receptors, signal 2 consisting of co-stimulatory molecules and signal 3 provided by cytokines. The potential of aAPCs is shown by recent progress of nanosized filaments to stimulate T cells. These nanosized filaments, consisting of a poly (isocyano peptide) co-polymer, were functionalized to attach signalling molecules that elicited strong sustained T cell activation in vitro [8, 89] (Figure 2). Future studies will most likely be directed to unravel the mechanisms of T cell activation by aAPCs, so design can be optimized to obtain full control over T cell response.
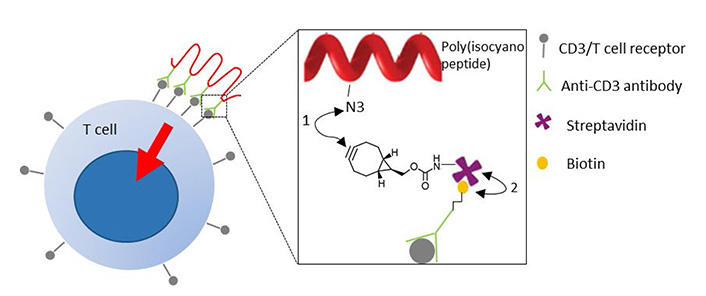
Artificial antigen presenting cells to activate T cells. Poly (isocyano peptide) is conjugated to an anti-CD3 antibody that activates T cell signalling upon binding T cell receptors. (1) Azide groups of poly (isocyano peptide) were coupled to bicyclononyne-functionalized streptavidin through a strain-promoted azide-alkyne click reaction (SPAAC); (2) streptavidin binds to biotinylated anti-CD3 antibodies
Another strategy in immunotherapy exploits light to kill cancer cells. Phototherapy has long been known for its cytotoxic effects, but only in the last decade the combination of light with chemicals are used to treat cancer [90]. In conventional PDT, a photosensitizing agent becomes activated on excitation by light. As it falls back to the ground state, the activated photosensitizer transfers energy to oxygen molecules. This leads to the generation of singlet and reactive oxygen species that mediate tissue damage [90].
Because PDT also evokes damage in healthy tissue, a targeted photosensitizer has been developed. In this approach, known as PIT, a monoclonal antibody is conjugated to a photosensitizer that is excited by NIR light (Figure 3). Mitsunaga et al. [7] conjugated the photosensitizer IR-700 to the HER1 receptor targeting antibody panitumumab. Upon excitation, panitumumab-IR-700 induced targeted specific cell death. In vivo injection of panitumumab-IR-700 and subsequent irradiation by NIR light, led to a significant decrease in volume of HER1 positive tumors [7].
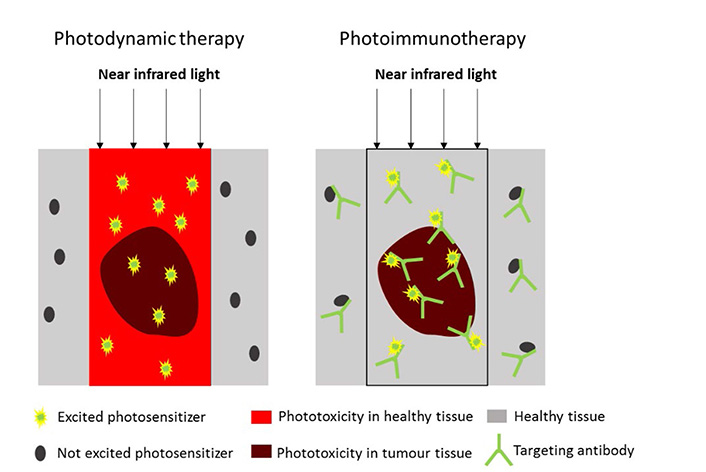
Difference between PDT and PIT. In PDT, a photosensitizer induces widespread tissue damage upon excitation by NIR light (left). In PIT, the photosensitizer is attached to a monoclonal antibody that specifically targets molecules expressed on cancer cells. Upon excitation, the photosensitizer only induces cell death when bound to the cell membrane (right)
Since the first success of PIT, also other targets have been used, including EGFR, mesothelin (hYP218) and prostate specific membrane antigen (PSMA) [91–94]. These studies also showed positive results with decreased tumor size and prolonged survival in vivo. A phase 2 clinical trial on the EGFR targeting cetuximab-IR-700 conjugate (RM-1929) is currently ongoing in the USA to determine efficacy in head and neck cancer [95].
A recent study indicates that the effect of PIT largely depends on induction of anti-tumor immunity. PIT rapidly caused damage that led to disposal of antigens and immunogenic signals, which was followed by maturation of dendritic cells [96]. This order of events is known to be associated with an anti-tumor response. However, no direct evidence was provided for the role of the host immune system in the effectivity of PIT. Studies in immunodeficient mice might elucidate the exact contribution of the host immune system in the effect of PIT [97]. Since immune escape mechanisms are an important hallmark of cancer, the application of nanoparticles to stimulate host immunity to cancer may lead to high therapeutic response.
Debates about the hype or hope of nanomedicine are ongoing and represent the controversy in the field. Nanomedicine might have suffered from high expectations, causing skepticism after lagging results. Yet, we cannot deny the first promising results. The ability to improve pharmacokinetics and modify properties has potential to increase drug efficacy, while limiting side effects in patients. Some nanomedicines have already shown clinical success, but the application in daily practice is still in its infancy. Despite the emergence of a tremendous number of designs for nanoparticle-based drug delivery, only a few are approved for clinical use. It is essential to understand the underlying reasons for this poor clinical translation to address remaining challenges.
High failure rates of nanomedicine can be partly attributed to unforeseen interactions in the body that cause limited specific uptake and high toxicity. It is therefore of utmost importance to understand the effects of different physicochemical properties on the behavior in biological environments. These insights are also necessary to improve design. However, the combination of design considerations to generate targeted, effective and also safe nanomedicine, is complex due to the interdependence of different properties and main functionalities. PEGylation might for example increase drug circulation time, but inhibits cellular uptake and endosomal escape [29, 98]. To overcome this phenomenon, known as the ‘PEG dilemma’, various nanocarriers have been developed that cleave PEG upon stimuli [99, 100]. The PEG dilemma emphasizes the need to overcome possible detrimental effects of beneficial properties. Small differences in properties such as size, charge and PEG density could have large effects on biodistribution and uptake [101–103]. Therefore, properties of nanoparticles should be accurately characterized and described. Two techniques that are commonly used to measure size and charge of nanoparticles are dynamic light scattering and zeta potential but concerns have been raised regarding their use and data interpretation [104]. Variation in data quality between studies makes it difficult to interpret and reproduce results. Therefore, it is important to standardize protocols for nanoparticle characterization. This could substantially increase the understanding of how nanoparticle characteristics influence interactions in the body, which is essential knowledge to improve design.
Another difficulty in design, arises from the demand to cross multiple different barriers in the body. Nanoparticles need to have different functionalities in each different environment. Clearance of nanoparticles should first of all be avoided to reach the tumor vasculature, whereas leakage to tumor tissue and subsequent accumulation depend on the EPR effect. Free drugs should then reach the target, either by release after cellular uptake or by diffusion through the tumor microenvironment. The final drug concentration at the target site is highly dependent on the efficiency of cellular uptake and controlled drug release. Knowledge about the nanoparticle-cell membrane interface and subsequent internalization pathways is inevitable to predict potential nanomedicine efficacy and toxicity, and to promote clinical translation.
All the desirable effects should be considered to generate effective nanomedicine but may be challenging to combine. Nanoparticle design will be facilitated by knowledge from other disciplines such as cell biology and cancer pathophysiology. The importance of this biological knowledge for nanomedicine design has already been illustrated by considering the particle surface to prevent opsonization or increase uptake, the size to allow the EPR effect, controlled drug release by triggers in the tumor microenvironment, and the influence of membrane composition on cellular uptake. Although these insights can improve basic design considerations in general, there is no ‘one size fits all answer’. Because of the highly heterogeneous nature of cancer, personalized strategies will become important to achieve optimal therapeutic efficacy in each individual.
The accumulation of nanoparticles in tumor tissue is attributed to the EPR effect, but disappointing clinical results have questioned the significance of this effect. The EPR effect is often exaggerated in animal models because of induced tumor growth and substantial differences from human cancer [105] (Figure 4). The extent of the EPR effect in human, is in fact highly heterogeneous between patients and cancer types [106]. For instance, hepatocellular carcinoma generally has a high EPR effect, whereas this effect is limited in pancreatic and prostate cancer because of low vascular density that leads to hypoxic areas. Furthermore, a high tumor tissue density leads to compression of blood vessels, which increases the interstitial fluid pressure which acts as a counter pressure in the extravasation of nanomedicine.
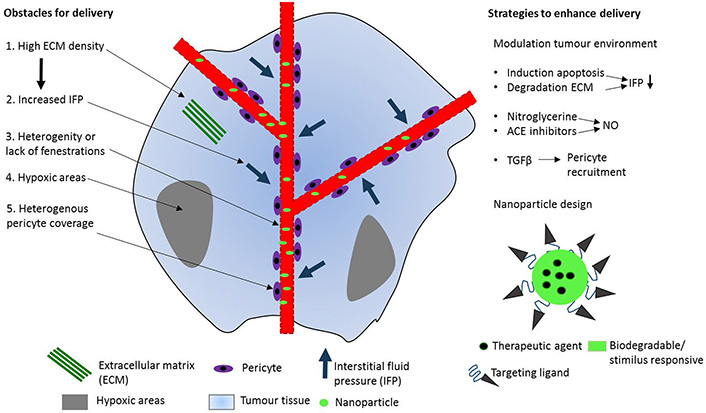
Obstacles and strategies in drug delivery to tumor tissue. Five factors in the human tumor microenvironment that limit nanomedicine delivery to tumor tissue (left). These five features are distinct from murine tumors and might therefore explain the lower drug delivery efficiency in humans when compared to animal models. Strategies to enhance the EPR effect by modulating the tumor microenvironment (upper right). Drug delivery can also be improved by modifying nanoparticles to increase cellular uptake and drug release at the target (lower right). ACE: angiotensin-converting enzyme; NO: nitric oxide; TGF: transforming growth factor
Modulating the tumor microenvironment to enhance the EPR effect is promising (Figure 4) [107]. Induction of tumor cell apoptosis or extracellular matrix (ECM) degradation in high density tumor tissues could lead to a decreased interstitial fluid pressure by vessel decompression. The cytotoxic drug paclitaxel has shown to increase the EPR effect in pre-clinical and clinical studies [108–110]. Induction of tumor cell apoptosis led to blood vessel decompression in vivo and also demonstrated decreased interstitial fluid pressure in breast cancer patients. The EPR effect can also be enhanced by increasing the vessel permeability. Agents that generate NO mediate vascular permeability and release local NO in hypoxic areas, resulting in and enhanced drug delivery by 2 to 3-fold [111]. Another strategy to increase vascular permeability is by targeting the pericyte coverage of tumor endothelium. TGF-β inhibitors increase vessel leakiness by inhibition of pericyte recruitment to tumor vessels and improved both delivery of 100 nm nanoparticles and anti-tumor activity [112].
The improved delivery by enhancing the EPR effect suggests that nanomotors might also have potential to facilitate accumulation in tumor tissue. However, for selective accumulation it is essential that these nanomotors move towards a specific tumor substrate. Non-specific uptake in healthy tissues is a common problem of nanoparticles in general, and often evokes toxicity. This is especially a concern in healthy tissues that also possess the EPR effect such as liver and spleen. To limit toxicity, nanoparticles and their biodegradation products should consist of biocompatible non-toxic materials. Toxicological assessments and research on their fate is important for safety analysis and can elucidate mechanisms of toxicity.
After nanomedicine accumulates in tumor tissue, cellular uptake is needed to evoke therapeutic effects. Active targeting can increase selective cellular uptake and is especially beneficial when drugs have intracellular targets. Moreover, active targeting has potential to circumvent drug resistance mechanisms [47]. However, its added value has been subjected to debate. Functionalized liposomes have been criticized for their increased clearance by the immune system [113]. When compared to passive targeting, the shorter circulation time of targeted nanomedicine might lead to less drug accumulation in tumor tissue. To clarify possible drawbacks of active targeting, studies should not only compare the efficacy of novel targeting approaches to free drugs, but also to their non-targeted counterpart. And although results are usually not generalized for different particles and cell types, it is important to realize that study outcomes might differ because of distinct uptake mechanisms. The type of endocytotic pathway is largely dependent on particle characteristics, targeting moiety, and cell type [114]. So extensive studies should be carried out for every new particle design or when the same nanoparticles are evaluated in a different cancer type.
Another issue that seems contradictory, involves the disappointing results of using targeting moieties with high avidity. High avidity nanoparticles are generally believed to be advantageous but are limited by their low tumor penetration [115]. Small antibody fragments and peptide ligands with weak affinity show higher tumor penetration, which is associated with higher efficacy [113]. Taken together, these results emphasize the need to consider effects on clearance and tumor penetration in the design of active targeting strategies.
The efficiency of active targeting not only depends on nanoparticle characteristics, but also largely depends on the target. The target should be highly abundant in tumor tissue, while expression in healthy tissue should be low. Molecular expression on cancer cells is highly heterogeneous within and between patients. It is therefore important to characterize cancer cells and identify appropriate targets. Nanomedicine can then be adjusted to each patient and different targets can be used to address heterogeneity within one patient. Moreover, nanotheranostic capsules could help to identify reasonable molecular targets and establish optimal dose. In addition to molecular targets, other interindividual distinctions in the tumor microenvironment, such as in vascularisation and ECM density, should also be considered to reinforce therapy (Figure 3). However, this requires careful tumor characterization, which may be challenging when biopsies are not possible.
The ineffective drug release is one of the major limitations of current nanomedicine and gave rise to controlled drug release systems. Various stimuli responsive nanoparticles have shown to increase selective drug release at the target [116]. Although it is useful to know more about the possibilities and working mechanisms of triggered drug release, many of these systems are not suitable for clinical use. For instance, heat triggered release might not be possible in human because of safety issues from increased temperatures. Moreover, the use of magnetic fields to trigger drug release might not be feasible in long-term treatment because of the inconvenience and high costs that are associated with the high frequency of patient exposure. Although frequency of nanomedicine administration might be kept to a minimum by prolonged and sustained drug release. Drug release by internal triggers from the tumor microenvironment might be more appropriate due to its use of natural triggers. However, this would require very sensitive systems that only release drugs on tumor specific stimuli. Co-triggered systems, such as pH/glutathione sensitive nanoparticles could therefore increase specificity of release. Nevertheless, heterogeneous responses can be expected because of interpatient differences in tumor environment.
Although more clinical success in cancer nanomedicine is known (reviewed in [117]), the amount of nanomedicine that reaches the clinic is low. Most progress can likely be achieved from taking into account clinical translation in fundamental research. Novel nanobased designs are increasingly emerging at a high pace but often neglect potential clinical application. For this purpose, it is important to design non-toxic adjustable nanoparticles with limited production requirements. These modular delivery platforms are essential to reduce costs and to enable bulk production. Another important aspect to decrease the translation gap, is the use of pre-clinical models that adequately resemble the human condition. Advanced in vitro models, such as tumors-on-a-chip, are promising to evaluate nanomedicine and could significantly facilitate clinical translation [118, 119].
Taken together, research has indicated the difficulty to achieve targeted, effective, safe, and at the same time, easy to produce nanomedicine. Many challenges lie ahead, but the promising results indicate great potential in personalized cancer treatment. Before nanomedicine can actually lead to a revolution in cancer therapy, a multidisciplinary approach should be adopted by combining knowledge from different disciplines including cell biology, tumor pathophysiology and chemistry. Besides in cancer therapy, nanomedicine tools have been developed for the treatment of several autoimmune and inflammatory diseases (reviewed in [120–122]). The lessons learned for the development of nanomedicine-based delivery strategies of immunomodulatory agents and anti-inflammatory molecules will certainly benefit the field of cancer and vice versa.
While this review mostly focused on polymeric nanoparticles, it is worth mentioning the development of prodrug based nanomedicines, which can controllably release drugs at specific sites under specific stimulation (see [123] for in-depth review). The so-called prodrug (i.e. the drug conjugated to a pre carrier) is an inactive compound that can be locally and controllably cleaved or degraded into the real bioactive drug directly in vivo [123]. Numerous prodrug-based nanomedicines with different chemical modifications have been developed that are responsive to both endogenous stimuli [124, 125] and exogenous environmental stimuli [126], thus expanding the repertoire of nanomedicines available to treat cancer.
Progress in nanomedicine would require a better understanding of nanoparticle behavior in biological environments, a more careful characterization to facilitate reproducibility, and increased focus on clinical application. Expanding our knowledge will facilitate improvements in design, ultimately leading to more efficient and less toxic drugs. Nanomedicine might then live up to its promise and substantially improve patient outcomes in the future.
aAPCs: artificial antigen-presenting cells
EGFR: epidermal growth factor receptor
EMA: European Medicines Agency
EPR: enhanced permeability and retention
FDA: Food and Drug Administration;
HA: hyaluronic acid
MDR: multidrug resistance
NIR: near-infrared
NO: nitric oxide
PDT: photodynamic therapy
PEG: polyethyleneglycol
PIT: photoimmunotherapy
PLGA: poly (D, L-lactide-co-glycolide)
siRNA: small interfering RNA
TNBC: triple-negative breast cancer
All authors contributed to manuscript writing and revision, read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2021.
Copyright: © The Author(s) 2021. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Jialu Xu, Chao Wang
Pooria Safarzadeh Kozani ... Mohammad Tariq Malik
Sureshbabu Ram Kumar Pandian ... Krishnan Sundar
Priyanka Singh ... Anita Kamra Verma
