Affiliation:
Section of Endocrinology, Diabetes and Metabolism, University and Azienda Ospedaliera Universitaria Integrata of Verona, 37126 Verona, Italy
Email: alessandro.mantovani@univr.it
Affiliation:
Section of Endocrinology, Diabetes and Metabolism, University and Azienda Ospedaliera Universitaria Integrata of Verona, 37126 Verona, Italy
Explor Med. 2020;1:42–50 DOI: https://doi.org/10.37349/emed.2020.00004
Received: November 22, 2019 Accepted: December 31, 2019 Published: February 29, 2020
Academic Editor: Yingyong Zhao, Northwest University, China
Chronic kidney disease (CKD) is a disease regularly seen in clinical practice. At present, CKD is described as a change of kidney structure and/or function and it is classified in relation to cause, values of glomerular filtration rate and albuminuria category. Seeing that CKD is closely linked to the development of end-stage renal disease and other comorbidities, the determination of additional independent predictors for CKD is clinically necessary. At present, there is evidence associating non-alcoholic fatty liver disease (NAFLD) with CKD, thereby suggesting that NAFLD patients may require intensive surveillance to reduce their risk of CKD. In 2008, genome-wide association studies documented an association between the variant rs738409 (C > G p.I148M) in the patatin-like phospholipase domain containing 3 (PNPLA3) gene (mainly implicated in the lipid regulation) and the entire spectrum of NAFLD (i.e., liver steatosis, non-alcoholic steatohepatitis, fibrosis, and hepatocellular carcinoma). In the last years, accumulating epidemiological evidence suggests the existence of a relationship between PNPLA3 rs738409 and risk of CKD, indicating that rs738409 may also contribute to the kidney injury. This is of particular scientific interest, as such association may explain, at least in part, the epidemiological association between liver and kidney disease. In this narrative review, we will discuss the accumulating evidence regarding the association between PNPLA3 rs738409 and risk of CKD, the putative biological mechanisms underpinning such relationship, and the possible future perspective.
Chronic kidney disease (CKD) is a progressive disease habitually seen in clinical practice [1]. It is currently described as an alteration of kidney structure and/or function and it is classified on cause, values of glomerular filtration rate (GFR) and albuminuria category [1]. It is estimated that the prevalence of CKD is approximately 13% among the adults of the general population worldwide [2]. In this regard, for instance, the US Renal Data System has documented that in 2014 roughly 670,000 adults received a renal replacement therapy [3]. Alarmingly, this number is believed to markedly rise by 2030/2050 [3]. Of note, the presence of CKD determines severe repercussions for multiple organs, seeing that it increases the risk of developing end-stage renal disease, cardiovascular disease and other serious comorbidities, thereby determining also a relevant increase in costs for the health systems [1, 4]. For these reasons, the appropriate identification of additional non-conventional independent predictors for CKD is essential.
The patatin-like phospholipase domain-containing protein-3 (PNPLA3) is particularly expressed on the lipid droplets of hepatocytes and is closely implicated in the development and progression of non-alcoholic fatty liver disease (NAFLD) [5–7], which is currently the most frequent chronic liver disease worldwide [8]. In this regard, it is important to highlight that NAFLD affects roughly 25% of adults, about 70% of patients with type 2 diabetes (T2DM) and virtually all obese individuals [8]. In the last decade, additionally, it is clearly demonstrated that NAFLD (as detected by imaging or liver biopsy) is associated with hepatic complications, but also with extra-hepatic complications, including cardiovascular disease [9]. The rs738409 C > G single nucleotide polymorphism, encoding for the I148M protein variant PNPLA3, is a key genetic determinant of the risk of NAFLD and its advanced forms, including non-alcoholic steatohepatitis (NASH), advanced fibrosis and hepatocellular carcinoma [5–7].
In the last 5–10 years, several observational reports have documented that imaging-diagnosed NAFLD is independently associated with an increased risk of developing CKD in patients with and without T2DM [10–23]. In addition, accumulating evidence now suggests that the rs738409 G allele is associated with lower values of estimated glomerular filtration rate (eGFR) as well as with a higher risk of CKD in adults but also in adolescents and children, even after adjustment for the presence of NAFLD and multiple CKD risk factors [13, 24–30]. This association may explain, at least in part, the epidemiological association between liver and kidney disease [10–23].
In our narrative review, we will discuss: (a) the accumulating evidence regarding the association between PNPLA3 rs738409 and risk of CKD, (b) the putative biological mechanisms underpinning such relationship, and (c) the possible future perspective.
The PNPLA3 rs738409 C > G is a genetic determinant closely linked to the entire spectrum of NAFLD across multiple different patient populations [5–7]. The PNPLA3 rs738409 was found as risk locus for NAFLD in 2008 through genome-wide association studies (GWAS) [31]. Subsequently, several experimental studies have suggested that PNPLA3 is an enzyme implicated in the lipid regulation with a triacylglycerol lipase and acylglycerol O-acyltransferase activity and a retinyl ester activity, especially in the hepatic stellate cells [32–36]. The biological mechanism for the development and progression of NAFLD seems to be related to the accumulation of the 148M mutated protein on the lipid droplet of hepatocytes, thereby determining relevant alterations of lipid remodeling and concurring to the liver injury [5–7].
Several variants in PNPLA3 gene have been also found by several GWAS as risk locus for hepatic cirrhosis due to alcohol abuse [5, 37, 38] or chronic infection by hepatitis B and C virus [5, 39, 40]. Of note, the PNPLA3(rs738409 C > G) is closely associated with severe steatosis and even liver carcinogenesis also in patients with alcoholic and non-alcoholic cirrhosis [5, 37–40]. Findings obtained in patients with HCV-related HCC remain, however, debatable [5].
Most studies reported that PNPLA3 is not linked with body mass index, lipids, plasma glucose levels or insulin resistance [5]. This aspect was also corroborated by a recent meta-analysis involving roughly 7,000 individuals with NAFLD [41]. In addition, although it is known that NAFLD is closely associated with an increased risk of T2DM, cardiovascular disease and CKD [8–13], data available so far suggest only a fair overlap in genome-wide significant associations [6, 42]. Specifically, some variants related to NAFLD risk, including rs738409, show divergent effects between the traditional metabolic alterations and the development of diseases, especially for cardiovascular complications [6, 42]. However, at present, accumulating observational data for rs738409 seems to indicate that this single nucleotide polymorphism might be associated to the presence of CKD [24–30].
Increasing evidence now supports the existence of an association between PNPLA3 G/G genotype and decreasing eGFR values or higher prevalence of CKD across different patient population, even after controlling for many CKD risk factors and for the presence of NAFLD [24–30] (Table 1). For instance, in a cross-sectional study of 740 Japanese individuals (about 16% of whom had NAFLD on ultrasonography), Oniki et al. [24], showed that patients with rs738409 G/G genotype had lower eGFR values than those with G/C or C/C genotypes, even after adjustment for multiple cardio-renal and metabolic risk factors. This association was further replicated in a longitudinal sub-analysis including roughly 350 non-obese patients followed for nearly 6 years [24]. In another cross-sectional study of approximately 200 Caucasian non-obese non-diabetic adults, Musso et al. [25], showed that rs738409 G/G genotype was linked to lower values of eGFRCKD-EPI as well as to higher levels of abnormal albuminuria. Additionally, in a recent cross-sectional study of nearly 100 Caucasian post-menopausal women with T2DM (approximately 44% of whom had NAFLD as detected by fatty liver index ≥ 60), Mantovani et al. [26], showed that rs738409 G/G genotype was linked to lower eGFRCKD-EPI values and higher risk of prevalent CKD, even after adjustment for several cardio-metabolic risk factor and the presence of NAFLD. Recently, in a cross-sectional study of 227 Chinese adults with NAFLD on histology, Sun et al. [27], reported that PNPLA3 GG genotype was associated with a higher risk of prevalent CKD, abnormal albuminuria or higher levels of urinary neutrophil gelatinase-associated lipocalin (a new marker of renal tubular injury), regardless of age, sex, hypertension, T2DM and severity of NAFLD.
Observational studies on the relationship between patatin-like phospholipase domain-containing protein-3 rs738409 genotype and kidney function (ordered by publication year and study population)
| Author, Reference | Study characteristics | Diagnosis of NAFLD | PNPLA3 rs738409 genotypes | Glomerular filtration rate formulas | Statistical adjustments | Main results |
|---|---|---|---|---|---|---|
| Adults | ||||||
| Oniki et al. [24] | Cross-sectional and retrospective longitudinal studies: 740 and 393 Japanese participants (followed for 5.5 years) respectively, during a health screening program | Utrasonography | G/G: n = 139 patients; G/C: n = 399 patients; C/C: n = 202 patients | Japanese eGFR equation | Age, gender, body mass index; diabetes, hypertension, dyslipidemia, fatty liver | Carriers of G/G genotype and normal weight had reduced eGFR levels than those with C/C or C/G genotypes |
| Musso et al. [25] | Cross-sectional study: 202 non-obese and non-diabetic individuals (61 with biopsy-confirmed NAFLD) | Biopsy | G/G or G/C: 112 patients; C/C: n = 90 patients | Chronic kidney disease epidemiology (CKD-EPI) collaboration equation | Age, gender, body mass index; metabolic syndrome | Carriers of G/G or C/G genotypes were linked to higher risk of albuminuria and CKD than C/C genotype |
| Mantovani et al. [26] | Cross-sectional study: 101 Caucasian post-menopausal women with type 2 diabetes | FLI ≥ 60 (ultrasonography in a subset of patients) | G/G: n = 8 patients; G/C: n = 41 patients; C/C: n = 52 patients | CKD-EPI collaboration equation | Age, diabetes duration, hemoglobin A1c, insulin-resistance, systolic blood pressure, hypertension treatment, FLI | Carriers of G/G genotype had reduced eGFR levels and higher prevalence of CKD than to C/C or C/G genotypes |
| Sun et al. [27] | Cross-sectional study: 227 Chinese patients with NAFLD | biopsy | G/G: n = 14 patients; G/C: n = 31 patients; C/C: n = 30 patients | CKD-EPI collaboration equation | Age, sex, body mass index; waist circumference, hyperuricemia, insulin-resistance, hypertension, diabetes, NASH, liver fibrosis | Patients with NAFLD and normal liver enzymes, who carried the PNPLA3 rs738409 G allele, were at higher risk of glomerular and tubular injury |
| Children and adolescents | ||||||
| Targher et al. [28] | Cross-sectional study: 142 Caucasian children and adolescents with NAFLD | Biopsy | G/G: n = 45 patients; G/C: n = 56 patients; C/C: n = 41 patients | Bedside Schwartz equation | Age, sex, systolic blood pressure, measures of adiposity, insulin-resistance, NASH, liver fibrosis | Carriers of G/G genotype had reduced eGFR levels and higher proteinuria than C/C or C/G genotypes |
| Marzuillo et al. [29] | Cross-sectional study: 591 Caucasian obese children | Ultrasonography | G/G: n = 87 patients; G/C: n = 219 patients; C/C: n = 285 patients | Bedside Schwartz equation | Gender, duration of obesity, alanine transaminase, insulin resistance, lipids | Carriers of G/G genotype had reduced eGFR levels than those with C/C or C/G genotypes |
| Di Costanzo et al. [30] | Cross-sectional study: 230 Caucasian overweight/obese children | Magnetic resonance imaging | G/G: n = 22 patients; G/C: n = 100 patients; C/C: n = 108 patients | Bedside Schwartz equation | Age, sex, pubertal status, waist circumference, diastolic blood pressure, NAFLD | Carriers of G/G genotype did not have lower eGFR levels than those with C/C or C/G genotypes |
FLI: fatty liver index
These findings were also replicated in some cohorts of children and adolescents [28, 29], although not in all [30]. For instance, in a sample of nearly 140 overweight children with NAFLD on histology, Targher et al. [28], reported that rs738409 G/G genotype was independently associated with both decreasing e-GFR and increasing 24 h urinary protein excretion. Another cross-sectional study involving 591 Caucasian children with obesity, it was documented that those with G/G genotype had significantly lower eGFR levels than those with G/C or C/C genotypes [29]. Conversely, in a recent cross-sectional study of 230 Caucasian overweight/obese children, Di Costanzo et al. [30], found that children with G/G genotype did not have lower eGFR levels than those with C/G or C/C genotypes. However, as recognized by the same authors, it is possible to speculate that the relatively low frequency of PNPLA3 G/G genotype in that study and the inclusion of children with and without NAFLD may provide a neutral relationship between NAFLD, PNPLA3 rs738409 and renal function [30].
Collectively, however, these observational data supported the notion that rs738409 G/G genotype is associated with an increased risk of CKD. This association seems to remain statistically significant in patients with and without T2DM even after controlling for several CKD risk factors and the presence of NAFLD. However, some relevant aspects of the aforementioned observational studies should be mentioned here. First, all studies used different creatinine-based GFR estimating equations. We believe that the use of direct measurements of GFR would have been more appropriate, as it is known that the equations based on serum levels of creatinine might be not accurate in estimating GFR in some specific patients, including those with obesity or advanced liver disease [13]. Second, NAFLD was diagnosed by imaging techniques or specific indirect markers and, rarely, by histology (which is the “gold standard” for the diagnosis of NAFLD [8, 9]). Third, no further information was available regarding the renal pathology. Fourth, at present, no information regarding the association between PNPAL3 rs738409 and CKD is available in non-NAFLD patient cohorts. We believe that this aspect should be timely investigated in order to corroborate the accumulating evidence regarding the potential effect of rs738409 on the kidney function. Lastly, PNPLA3 variant in association to CKD has not been identified by GWAS studies yet.
To date, the mechanisms underpinning the relationship between PNPLA3 rs738409 and impaired kidney function are poorly understood. In the literature, it is still discussed whether the G allele of rs738409 exerts a direct adverse impact on kidney function or whether it indirectly influences the kidney function by hepatic lipid status. Seeing that the presence of NAFLD is associated to unfavourable metabolic profile with the subsequent decline of renal function, Marzuillo et al. [29], have speculated on the existence of a vicious circle in which the PNPLA3 148M allele may predispose individuals to NAFLD, and, in turn, NAFLD may boost the (adverse) effect of the PNPLA3 148M allele on renal function. Corroborating this hypothesis, Pirazzi et al. [35], performed an in vitro study reporting that PNPLA3 was expressed in adipose tissue, but also in the kidneys. In addition, Hoekstra et al. [43], reported that the expression of PNPLA3 gene in the adipose tissue of mice was approximately 50-100-fold higher than that observed in the liver tissue. Interestingly, in that study, the difference in expression of PNPLA3 gene between adipose and liver tissue was increased when mice were fed with Western-type diet (which can be considered a condition of lipid excess), thereby suggesting that adiposity may amplify the genetic impact of PNPLA3 [43]. In this context, as reported by Stender et al. [44], it is important to note that the gene-adiposity interaction may have a relevant role in the development and progression of NAFLD. In line with this view, some authors have speculated that in the kidney the expression of PNPLA3 may be enhanced in condition of lipid excess, leading to the accumulation of lipids in podocytes but also in renal mesangial or tubular cells [25]. In this context, it is important to note that the accumulation of lipids in the kidney cells may also promote insulin resistance, oxidative stress and pro-inflammatory state, thereby leading to renal structural and functional alterations that may additionally weaken the integrity of glomeruli [45] (Figure 1).
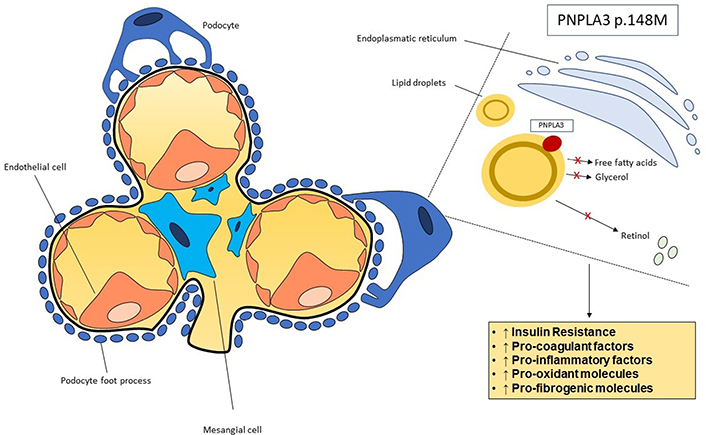
Putative mechanisms underpinning the association between PNPLA3 rs738409 genotype and kidney function. PNPLA3 has a hydrolase activity regarding triglycerides and retinyl esters. It is thought that in mesangial cells and podocytes PNPLA3 might be located in the lipid droplets. The I148M mutation leads a diminution of function of the protein with an excessive internment of fat and retinol in cells. The accumulation of lipids and retinol in the kidney cells may promote insulin resistance, oxidative stress and pro-inflammatory cytokines, thus resulting in renal structural and functional alterations
Fat accumulation and lipotoxicity may not be the only mechanisms proposed to influence the direct or indirect action of PNPLA3 on the impaired kidney function. Romeo et al. [46], for instance, speculated that the G variant of rs738409 may influence the glomerular filtration through an activation of kidney pericytes, thereby concurring to the development of kidney fibrosis. At present, indeed, it is believed that kidney fibrosis (along with chronic inflammation) is one of most important pathological process implicated in CKD [1, 12, 46]. Future studies are needed to elucidate the biological mechanisms underlying the relationship between PNPLA3 rs738409 and impaired kidney function.
Seeing that several epidemiological studies have documented that NAFLD is independently associated with higher risk of CKD, it remains to establish whether PNPLA3 variants are active contributors or innocent bystanders. Indeed, the issue of whether G allele risk is a strong mediator of the relationship between NAFLD and CKD becomes more and more apparent. The Mendelian randomization approach will help to make causal inferences.
At present, there are not formal recommendations for patients with several specific gene variants that predispose to NAFLD and its hepatic or extra-hepatic complications, including CKD. However, it is reasonable to assume that future and potential therapeutic strategies focused on personalized medicine will include PNPLA3 genetic classification test [13]. Although many clinical trials are still ongoing to assess the individual response to NAFLD intervention in function of gene polymorphisms, accumulating data now suggest that 148M carriers may respond differently to lifestyle and drug intervention [6, 47]. Therefore, it is likely that therapeutic interventions that decreased PNPLA3 I148M levels may ameliorate the severity of NAFLD and the consequences of its complications.
CKD is closely associated with all-cause mortality as well as with the development of end-stage renal disease, cardiovascular disease or other serious comorbidities [1]. Hence, the identification of further independent predictors for CKD is crucial in clinical practice. At present, there is strong evidence linking NAFLD and CKD, indicating that patients with NAFLD could need more intensive surveillance and treatment in order to reduce their risk of developing CKD [8–22]. Additionally, accumulating data also suggest the existence of an association between PNPLA3 rs738409 gene variant (which is strongly linked to the development of NAFLD and its advanced forms) and risk of CKD [23–29], thereby suggesting that PNPLA3 may directly influence the kidney injury [44]. This may also clarify the epidemiological association between liver and kidney disease. The pathophysiological mechanisms linking PNPLA3 gene, NAFLD and CKD seem to be complex and require additional studies to be elucidated. The identification of such mechanisms may result in novel therapeutic targets for the treatment of CKD. Meanwhile, it is reasonable to suppose that patients with NAFLD should be also better genotyped, given the potential role of the PNPLA3 gene on the risk of NAFLD and CKD.
CKD: chronic kidney disease
eGFR: estimated glomerular filtration rate
FLI: fatty liver index
GFR: glomerular filtration rate
GWAS: genome-wide association studies
NAFLD: non-alcoholic fatty liver disease
NASH: non-alcoholic steatohepatitis
PNPLA3: patatin-like phospholipase domain containing 3
T2DM: type 2 diabetes
AM and CZ contributed conception and design of the study; AM and CZ wrote the first draft of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2020.
Copyright: © The Author(s) 2020. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
