Affiliation:
Biomedical Informatics Lab, Department of Applied Sciences, Indian Institute of Information Technology Allahabad, Prayagraj 211015, Uttar Pradesh, India
Email: rss2020502@iiita.ac.in
ORCID: https://orcid.org/0009-0002-2722-9043
Explor BioMat-X. 2026;3:101356 DOI: https://doi.org/10.37349/ebmx.2026.101356
Received: September 18, 2025 Accepted: January 03, 2026 Published: January 25, 2026
Academic Editor: Amir Sanati Nezhad, University of Calgary, Canada
Hydrogels are among the most intensively studied biomaterials for controlled drug delivery, yet translation to routine clinical practice has been limited by rapid diffusion of small molecules and instability of biologics. In their recent report in Nature Nanotechnology (Pogostin et al., 2025, DOI: 10.1038/s41565-025-01981-6), a team from Rice University and collaborators present a nanofibrous supramolecular peptide hydrogel system that addresses these challenges through the incorporation of dynamic covalent chemistry. The SABER (Self-Assembling Boronate Ester Release) platform introduces reversible boronate ester bonds between engineered peptide fibers and boronic acid modified therapeutics, creating a tunable and long-acting drug release system. Proof-of-concept applications included tuberculosis therapy, diabetes management, and prolonged antibody delivery, demonstrating both versatility and clinical relevance. In this Commentary, I situate this advance within the broader trajectory of hydrogel research, highlight the conceptual novelty of dynamic supramolecular interactions, and discuss the opportunities and challenges for clinical translation. I argue that this platform signals a paradigm shift in drug delivery, moving hydrogels from passive depots to dynamic partners in medicine.
Since their emergence in the 1970s, hydrogels have been viewed as promising platforms for sustained drug release due to their high water content and biocompatibility [1–3]. However, despite progress in design, most systems permit rapid drug diffusion, particularly for small molecules, limiting their clinical value [4–6]. Biologics, although larger in size, may also suffer from reduced stability, including denaturation or loss of bioactivity, depending on hydrogel composition, crosslinking chemistry, and local microenvironmental conditions [7, 8]. These shortcomings have left the field searching for materials that can provide reliable, tunable, and patient-friendly drug delivery.
Self-assembling peptide materials have gained remarkable attention as biomimetic scaffolds for drug delivery over the past two decades. Their amphiphilic or β-sheet-forming motifs enable nanofiber formation and encapsulation of a wide range of therapeutics, from small hydrophobic molecules to proteins and nucleic acids [9–12]. These systems combine molecular precision with injectable softness, mimicking the extracellular matrix. Yet, because their interactions are primarily non-covalent, hydrogen bonding, π-stacking, or electrostatics, release rates are often dictated solely by diffusion and gel erosion. These limitations motivated the integration of reversible covalent interactions, such as boronate ester linkages, to achieve greater control over release kinetics, as exemplified by the SABER (Self-Assembling Boronate Ester Release) platform.
In this direction, Pogostin and colleagues introduce a supramolecular approach that marks a departure from passive hydrogel strategies [13]. Their system, SABER, is based on multidomain peptides that form β-sheet nanofibers, while carrying boronate reactive motifs. When combined with boronic acid, bearing drugs or proteins, reversible boronate ester bonds are formed. This interaction transiently anchors the therapeutic payload, slowing its release but ensuring eventual bioavailability.
Mechanistically, the SABER network regulates release through reversible boronate ester formation between boronic-acid-bearing therapeutics and hydroxyl-bearing side chains on the peptide nanofibers. These linkages continuously exchange between bound and free states, establishing an equilibrium that modulates the effective diffusion coefficient of the drug. In contrast, traditional hydrogels lacking dynamic bonding permit free Fickian diffusion and exhibit rapid burst release. The tunable equilibrium constant (K_eq) of the boronate ester, sensitive to pH and diol configuration, provides molecular-level control over retention time (Figure 1) [4, 14]. Importantly, the modification did not disrupt the hydrogel’s nanofibrous architecture or rheological properties, preserving injectability and tissue compatibility.
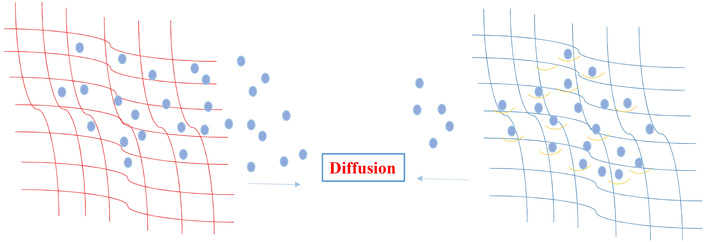
Comparison of conventional hydrogel versus SABER hydrogel release mechanisms. Left-side: Conventional hydrogel showing rapid diffusion of drug molecules through static polymer mesh. Right-side: SABER peptide hydrogel (teal) incorporating boronate-reactive motifs (yellow hooks) reversibly binding boronic-acid-modified drugs (purple). The reversible covalent interactions slow diffusion, enabling long-acting controlled release. SABER: Self-Assembling Boronate Ester Release.
A key mechanistic advantage of the SABER platform lies in the dynamic covalent nature of boronate ester interactions. Boronic acid modified therapeutics form reversible covalent bonds with the hydrogel’s boronate reactive motifs, creating a bound and unbound equilibrium within the matrix. This dynamic exchange reduces the effective diffusivity of the drug by repeatedly tethering and releasing the payload, thereby producing a controlled, sustained-release profile. In contrast, traditional peptide or polymer hydrogels rely primarily on static physical entrapment or noncovalent interactions, which cannot modulate drug residence time once diffusion begins. The tunability of the SABER system arises from the pH-dependent binding affinity of boronate esters, the density of reactive motifs on peptide fibers, and the chemical design of the payload itself, collectively enabling precise control over release kinetics [4, 13, 14].
The translational potential of SABER hydrogels is underscored by their performance in vivo in a model organism. In a murine model of tuberculosis (TB), a single SABER injection of ganfeborole achieved superior bacterial suppression compared with multiple oral doses, directly addressing adherence challenges that hinder global TB control [13]. Following the same approach and materials, the SABER hydrogel delivered boronic-acid-modified insulin to diabetic mice, maintaining normoglycemia for six days, an extraordinary extension relative to conventional insulin injections. Antibodies, notoriously difficult to deliver in sustained formats, were retained in hydrogels for weeks, highlighting the versatility of the approach. These demonstrations exemplify the potential of dynamic covalent chemistry in biomaterials design for precision drug delivery. Beyond the experimental demonstrations, the SABER study provides a conceptual advance by showing that drug pharmacokinetics can be engineered through reversible chemistry embedded within a supramolecular matrix. This shifts the design paradigm from optimizing polymer mesh size to tailoring molecular interactions that dictate residence time. Such a mechanistic framework opens new avenues for designing precision depots adaptable to multiple therapeutic classes.
Dynamic boronate ester chemistry provides several advantages for therapeutic delivery. These reversible bonds form rapidly and selectively between boronic acids and cis-diols under physiological conditions, enabling payloads to transition between ‘bound’ and ‘free’ states. This dynamic equilibrium slows diffusion and permits tunable release kinetics, distinguishing SABER from hydrogels that rely solely on physical entrapment [4, 14–16]. Additional benefits include chemical modularity, pH-responsive behavior, and compatibility with a wide range of small molecules and biologics that can be modified with boronic acid groups. However, boronate ester interactions also present limitations. Their stability may be reduced in acidic or oxidative environments, and some therapeutic molecules may not tolerate boronic acid functionalization without altering structure or bioactivity. Furthermore, variations in in-vivo diol concentrations, particularly in inflamed or pathological tissues, may influence bond stability, affecting release behavior, further complicating the predictability of long-term release kinetics. These characteristics underscore the need for careful molecular design and comprehensive in-vivo evaluation.
Whereas conventional hydrogels rely on static crosslinking or entrapment [9, 17, 18], dynamic boronate ester bonds provide reversible, tunable interactions. This aligns with broader trends in “smart” biomaterials, where materials adapt to biological environments and therapeutic needs [10, 11, 19]. The SABER platform thus situates itself at the intersection of supramolecular chemistry and clinical pharmacology, pointing to a future where biomaterials actively mediate drug pharmacokinetics.
Collectively, the in-vivo demonstrations by Pogostin et al. [13] highlight the practicality of dynamic supramolecular hydrogels for addressing long-standing adherence issues in chronic and infectious diseases. The concept bridges material chemistry and clinical pharmacology, offering a blueprint for rational design of programmable depots. Building on this foundation, future research could expand beyond boronic acid chemistry toward orthogonal reversible motifs, such as imine, disulfide, or hydrazone linkages, to broaden payload compatibility and fine-tune release kinetics. Long-term biodistribution and immunogenicity studies will be critical to translate these laboratory successes into clinical protocols.
The broader implications of this work extend across global health, chronic disease management, and immunotherapy [20, 21]. Thus, from a global health standpoint, a single injection that provides weeks of therapy could transform treatment adherence, particularly in resource-limited settings. For chronic diseases such as diabetes and arthritis, reducing dosing frequency could improve quality of life and healthcare efficiency [22, 23]. In oncology and immunotherapy, where biologics and checkpoint inhibitors often require repeated infusions, extended dosing intervals could reduce toxicity and costs [24, 25].
These limitations have meaningful implications for clinical translation. For example, boronic acid functionalization is not universally compatible with all small molecules or biologics; certain therapeutic structures may be destabilized or exhibit altered potency following modification. Moreover, physiological environments with fluctuating pH levels or oxidative stress may weaken boronate ester stability. Long-term implantation studies are therefore essential to assess the durability, immune tolerance, and biodegradation behavior of boronate-containing peptide materials. To address these challenges, future research may explore the incorporation of dual-dynamic chemistries, combining boronate esters with orthogonal reversible linkages such as hydrazone, disulfide, or imine bonds to enhance robustness. Designing BA-mimetic linkers that maintain strong affinity at physiological pH, or applying protective hydrophobic motifs around boronate sites, may further improve stability. On a translational level, modular peptide synthesis strategies and scalable solid-phase manufacturing pipelines will be critical for advancing SABER hydrogels toward regulatory approval and clinical-grade reproducibility [4, 14, 26, 27].
SABER: Self-Assembling Boronate Ester Release
TB: tuberculosis
DC: Conceptualization, Investigation, Writing-original draft, Writing-review & editing. The author read and approved the submitted version.
The author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2026.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2026. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 444
Download: 11
Times Cited: 0
