Affiliation:
1The Evington Centre, Leicester General Hospital, LE5 4QG, Leicester, UK
2Department of Psychology and Vision Sciences, University of Leicester, LE1 7RH Leicester, UK
Email: eml12@le.ac.uk
ORCID: https://orcid.org/0000-0003-0817-7591
Affiliation:
2Department of Psychology and Vision Sciences, University of Leicester, LE1 7RH Leicester, UK
ORCID: https://orcid.org/0000-0003-0334-213X
Affiliation:
2Department of Psychology and Vision Sciences, University of Leicester, LE1 7RH Leicester, UK
ORCID: https://orcid.org/0000-0002-0391-0187
Affiliation:
2Department of Psychology and Vision Sciences, University of Leicester, LE1 7RH Leicester, UK
ORCID: https://orcid.org/0000-0002-6849-6685
Explor Neuroprot Ther. 2023;3:457–469 DOI: https://doi.org/10.37349/ent.2023.00062
Received: June 28, 2023 Accepted: September 26, 2023 Published: December 22, 2023
Academic Editor: Masaru Tanaka, University of Szeged (ELKH-SZTE), Hungary
The article belongs to the special issue Defending the Brain and the Mind: Exploring Neuroprotective Therapies for Mental Health Disorders
The currently available pharmacological anti-dementia treatments provide only temporary and limited benefits. Not surprisingly, patients and professionals increasingly explore non-pharmacological interventions that may alleviate dementia symptoms. Among these interventions is hyperbaric oxygen therapy (HBOT). A brief review is presented on HBOT use in medicine, with its mode of action in dementia, specifically Alzheimer’s disease, as well as a case report of self-initiated HBOT in a 62-year-old man with a clinical diagnosis of probable Alzheimer’s disease. He had over 400 HBOT sessions [2–3 times weekly, with a duration of 30–50 min, in a multi-place hyperbaric chamber at 2 atmospheres absolute (ATA)] over 7 years and use of donepezil (10 mg daily) for the last 3 years when formally diagnosed by the National Health Service (NHS) Memory Service. The patient’s longitudinal neurocognitive and neuroradiological evidence over 7 years of follow-up remained stable (with no major cognitive decline and no behavioral changes) when compared to his initial presentation when diagnosed by the private health provider. His driving remains unimpaired, and he continues to be independent. This highlights the potential HBOT benefits including those on visuospatial ability and activities of daily living in people with Alzheimer’s disease. This case report argues for more extensive research into the clinical effects of HBOT in Alzheimer’s disease. Discussion of HBOT use is along with the latest advances in anti-amyloid immunotherapy for Alzheimer’s disease, as well as HBOT augmentation of current and novel dementia drug delivery via nanotechnology.
The pace of population ageing is much faster than in the past. Thus, only 3 years ago, the World Health Organization [1] reported that people over the age of 60 years have outnumbered children younger than 5 years of age. Furthermore, people over the age of 60 years are expected to account for at least one-fifth of the world population by 2050. Living older does not necessarily mean having a healthy life [2]. Thus, sensory changes, physical and mental frailty, cardiovascular diseases and diabetes, osteoarthritis, osteoporosis, and falls appear to be frequent in older people and are more pronounced above the age of 85 years [3]. These, alongside accompanying polypharmacy, especially anticholinergic drugs [4], all have a negative impact on activities of daily living [5], leading to many older people requiring support from formal and informal carers [6].
Dementia is the seventh leading cause of death among older people globally, affecting over 55 million people worldwide [7], with the majority of them (60%) living in low- and middle-income countries. It consists of over 100 types of different dementia types, depending on the causes and risk factors with distinct clinical symptoms and course. It is characterized by an impaired ability to remember, think, and/or make decisions, thus significantly affecting a person’s daily activities. In the latter stages of the disease progression, a range of so-called behavioral and psychological symptoms of dementia (BPSD; hallucinations, delusions, wandering, agitation, low mood, etc.) occur [8], and these are difficult to manage both at home and clinical environment and can cause distress to both patients and their carers. They also lead to carers’ burden and frequently necessitate 24 h care for people with dementia.
At present there is no cure for dementia. Although the identified potentially modifiable 12 risk factors (less education, hypertension, obesity, alcohol, traumatic brain injury, hearing loss, smoking, depression, physical inactivity, social isolation, diabetes, and air pollution) may help prevent or delay the dementia prevalence by 40% [9], the therapeutic options remain limited. Thus, the only recommended pharmacological treatments are those for Alzheimer’s disease (AD, the most common form of dementia affecting one in nine people and counting for 60–70% of all cases of dementia worldwide) and dementia with Lewy bodies, and then restricted to cognitive symptoms. The effect of these drugs [cholinesterase inhibitors, i.e. donepezil, rivastigmine, and galantamine, and an N-methyl-D-aspartate (NMDA) receptor antagonist, i.e. memantine] is rather modest and they only temporarily delay cognitive deterioration. In some instances, they can also help some of the dementia non-cognitive symptoms (i.e. aggression, agitation, wandering, hallucinatory, and delusional beliefs). Ruling out possible causes of distress (i.e. being in pain, dehydration, sensory deprivation, acute confusion as a result of underlying and unaddressed/undiagnosed infection, etc.) is the necessary first step prior to considering pharmacological intervention. These should be initiated and regularly reassessed (i.e. 6 weeks) only when people with dementia are at a high risk to harm themselves or others, or experience agitation, hallucinations, or delusions that are causing them severe distress [10]. However, antidepressant and neuroleptic medication used to regulate these BPSD can result in adverse side effects, such as sedation, parkinsonian features (extrapyramidal side effects), bradycardia, falls, and higher mortality rates, etc. [10].
The current limitation of dementia treatment, not surprisingly, calls for additional exploration of non-traditional medicines [11, 12]. Hyperbaric oxygen therapy (HBOT) is a non-pharmacological intervention and serves as either primary or adjunctive therapy in the management of potentially life-threatening conditions, such as carbon monoxide poisoning, decompression illness, and/or gas embolisms (Table 1) [13–17]. HBOT has also been found to improve attention, information processing speed, and executive functions [18], which normally decline with ageing. These improvements in cognition have now been demonstrated for dementia syndromes, in particular vascular dementia. Thus, a 12-week trial involving 158 patients with vascular dementia, all treated with donepezil 5 mg daily, reported that those with additional HBOT intervention had not only a significant improvement in cognition over the group treated with donepezil only but this was accompanied by an increase in blood humanin [19]. The authors hypothesized that the cognitive improvement was related to elevated serum humanin levels. Interestingly, humanin, a mitochondrial derived 24-amino acid secreted bioactive peptide, is widely distributed in blood vessel walls, neurons, skeletal muscle, and peripheral blood, and has anti-apoptotic, metabolic, and anti-inflammatory effects, and was first discovered in AD [20]. Vila et al. [21] described improvement in cognition (measured with the Mini Mental State Examination) and motor symptoms (as measured with gait and equilibrium scale, unified Parkinson’s disease rating scale, and Barthel scale) in their rather modest study on 18 people with vascular dementia who had only 10 days of HBOT treatments. Despite the short duration of the HBOT intervention, the neurological symptoms’ improvement (gait and motor symptoms) persisted in the majority of patients for up to 6 months. These results are similar to those reported in poststroke patients, which found significant neurological improvements even in those with chronic late-stage poststroke [22], suggesting that HBOT can stimulate neuroplasticity long after vascular neurological sequelae have occurred. An animal study demonstrated that HBOT improves the blood supply and promotes the neurogenesis in the piriform cortex (disruption of this region is known to mediate early olfactory deficits in AD) of adult rats with chronic cerebral hypoperfusion (an animal model for vascular dementia) [23]. The benefits of HBOT in vascular dementia are further supported by recent reviews [24, 25] and meta-analyses on non-pharmacological interventions in vascular dementia [26–28], which all reported a potential HBOT benefit on cognition and activities of daily living.
| Therapeutic benefits | Precautions needed* | Absolute contraindications | Side effects |
|---|---|---|---|
Decompression sickness** Arterial gas embolism** Carbon monoxide poisoning and smoke inhalation** Wound healing (i.e. burns, fractures)** Anemia (severe anemia when blood transfusions cannot be used)** Crush injury** Gas gangrene** Radiation injury** Idiopathic (sudden sensorineural) hearing loss** Sudden and painless vision loss in one eye due to blockage of blood flow** Severe skin graft flap at risk of tissue death** Infections due to antibiotic resistant bacteria (soft tissue infections**, osteomyelitis**, necrotizing fasciitis, infective endocarditis) Autoimmune illnesses (multiple sclerosis, type 1 diabetes, rheumatoid arthritis, psoriasis/psoriatic arthritis and psoriasis vulgaris, systemic lupus erythematosus and associated acute macular neuroretinopathy, inflammatory bowel disease, and Hashimoto’s thyroiditis) Long COVID Brain abscess Traumatic brain injury and concussion Fibromyalgia Brain fog Addiction Cerebral palsy Alopecia HIV Migraine Sports injuries Stroke Mental health diseases [i.e. post-traumatic stress disease, obsessive-compulsive disorder, autism spectrum disorder, mood disorder (i.e. depression, postpartum depression, bipolar disorder, premenstrual mood disorder), anxiety (including adjustment disorder with anxiety, panic disorder, social anxiety), schizophrenia (including schizoaffective disorder) Dementia | Recent ear surgery or injury Cold or fever Uncontrolled hypertension Uncontrolled diabetes Certain types of lung disease may increase the risk of getting a collapsed lung (i.e. COPD, cystic fibrosis, and emphysema) Caution required for concurrent use of: doxorubicin (increasing doxorubicin-mediated cardiotoxicity; drug should be stopped 24 h before HBOT); disulfiram (by blocking superoxide dismutase) can increase the risk of oxygen toxicity, and result in seizures and pulmonary toxicity. The recommendation is not to use concurrently disulfiram with HBOT; cisplatin, a chemotherapy drug used to treat testicular, ovarian, bladder, head and neck, lung, and cervical cancer, with HBOT is a relative contraindication since cisplatin can impair wound healing and make the treatment futile; mafenide (a sulfonamide-type medication used as an antibiotic) can cause carbon dioxide production leading to acidosis | Untreated pneumothorax Restrictive airway disorders Simultaneous chemotherapy Use of bleomycin (distant use of bleomycin, > 6 months, is required). Pre-treatment evaluation with a physical examination, radiography, blood gas, and spirometry is necessary to determine if HBOT is safe | Temporary fatigue (due to the body adapting to enhanced oxygen saturation) Barotrumatic lesions Oxygen toxicity Confinement anxiety Claustrophobia Trauma to the middle ear Eye damage Lung collapse Low blood sugar Sinus problems (i.e. a runny or stuffy nose, mucus drainage, a sinus headache) Transient nearsightedness Seizures (convulsions, sensory disturbances, and fainting) |
COPD: chronic obstructive pulmonary disease; *: Advice to postpone a therapy session based on a current health situation; **: US Food and Drug Administration (FDA) approved HBOT treatments [17]
In contrast to the use of HBOT in vascular dementia [19, 27], clinical studies on HBOT implementation in AD are rather scarce, despite HBOT being described to target the four characteristic AD pathological processes. Namely, it improves brain vascular circulation, mitochondrial dysfunction, and endothelial biogenesis, and reduces amyloid burden and tau protein phosphorylation, oxidative stress, and inflammation (as reviewed in [29]). HBOT properties to reduce neuroinflammation via down-regulating proinflammatory cytokines [interleukin-1β (IL-1β), IL-12, tumor necrosis factor α (TNFα), and interferon-gamma (IFNγ)] while upregulating an anti-inflammatory cytokine (IL-10) [30] may provide neuroprotection directly via its cytoprotective properties, alternatively, via promoting further additional induction of cytoprotective molecules [i.e. heat shock protein 27 (Hsp27), peroxiredoxin 6 (Prdx6), apolipoprotein E (APOE), and latexin (LTXN)] all of which may function as a neuroprotective mechanism against tau toxicity in AD [31]. In transgenic mice with five familial AD mutations, HBOT increased arteriolar luminal diameter and elevated cerebral blood flow. In addition, this was accompanied by changes in amyloid precursor protein processing, elevated degradation and clearance of amyloid protein, and improved behavior in the AD transgenic mice [31]. This suggests that HBOT tackles tau and amyloid accumulation and processing differently, via stimulating and augmenting cerebral and regulating hypoxia cytoprotective molecules (hypoxia is known to increase amyloid processing via altering expression levels of enzymes involved in the production/degradation of the protein and neuronal and glial-cell dysregulated neurotransmitter signaling and intracellular calcium-store dysfunction, as reviewed in [32]), respectively.
In the current study, the use of HBOT as an adjuvant therapy in a patient with early onset AD is under discussion and the literature is review to inform about this non-pharmacological intervention and its incorporation in clinical settings. A review of HBOT’s current and future use is presented considering the most recent advances of modifiable AD therapy and nanomedicine.
The patient (62-year-old Caucasian man, University graduate) was diagnosed with mild cognitive impairment (MCI) at the age of 54. At that time, he was not aware of any problems with his memory, but his wife stated that he was forgetful for about one year, but without much evidence for progression. He tended to repeat himself, sometimes would forget what he had been told, would go to the shops to get one item, and would come back with several, not including the one he went shopping for. His current and previous medical history was uneventful, and he did not use prescription or recreative drugs that could have caused memory problems as side effects.
On the clinical assessment completed, he scored 91/100 on the Addenbrooke’s Cognitive Assessment III (ACE-III), losing 1 point on memory, 2 points on attention, and 6 points on visuospatial ability. His computerised tomography (CT) brain scan at that time showed mild global atrophy, with no typical features of dementia. The magnetic resonance imaging (MRI) brain scan done 6 months later showed possible bilateral atrophy of the parietal lobes, with no hippocampal atrophy and no evidence of cerebral vascular disease. The electroencephalogram (EEG) performed at the same time had normal background rhythms during wakefulness and light sleep, with mild excess of intermittent left fronto-temporal slow wave activity, indicating an area of mild dysfunction.
Two years later, at the age of 56, he was seen again by a neurologist, due to his more pronounced forgetfulness with him starting writing notes to remind himself, finding it harder to do familiar work. His behaviour seems to have changed and he was not concerned about his activities as he used to be. The repeated ACE-III score was 16 points less than the one he obtained two years before. Thus, he scored 75/100 due to losing points on memory, and word finding tasks. Antidementia drugs were not considered at this point. He himself accessed HBOT at that time on the advice of a peer and attended 2–3 times weekly sessions (with a duration of 30–50 min) in a multi-place hyperbaric chamber (2 ATA, which refers to two atmospheres absolute; this is twice the atmospheric pressure exerted at sea level) in the local hyperbaric centre.
Three years later, at the age of 59, he reported problems following and processing new information, problems with driving (e.g., observing traffic lights, following signs, satellite navigation, etc.), and communication, with ‘periods of (brain) fog’ and an inability to follow even familiar tasks, being ‘emotionally unbalanced’, and deteriorating memory. He was fearful of making mistakes, checking tasks repetitively, and reported having made one isolated error with the banking. His wife also noted difficulties in following instructions and differentiating between left and right. His mood, sleep, and appetite were unaffected, except for some frustration when failing to complete tasks satisfactorily. Both he and his wife attributed his most recent deterioration of memory, processing of information, and thinking to stopping HBOT for 12 weeks, as a result of the COVID-19 pandemic in early 2020. This also coincided with his early retirement, due to the pandemic. On assessment, in 2020, his speech was fluent with no obvious phonetic or semantic errors noted. He scored 77/100 on the ACE-III with a differential of attention 13/18, memory 20/26, fluency 8/14, language 24/26, visuospatial 12/14 subscales (Figure 1a). Physical and neurological examinations were unremarkable. Fluorodeoxyglucose-positron emission tomography (FDG-PET) brain scan showed a significantly reduced uptake in both parietal and temporal lobes and posterior cingulate gyrus with associated cortical sulcal widening. There was a moderately reduced uptake at the left lateral frontal lobe and reduced uptake at the precuneus, with preserved uptake in the occipital lobes. The neuroradiological examination, alongside the neurocognitive assessment and clinical history, was supportive of an intrinsic neurodegenerative disorder, namely AD. He was commenced on donepezil 5 mg daily, and the dose was increased after 6 months to 10 mg daily. In addition, he restarted HBOT thrice weekly hour-long sessions in which 20 min were spent with him being at oxygen saturation.
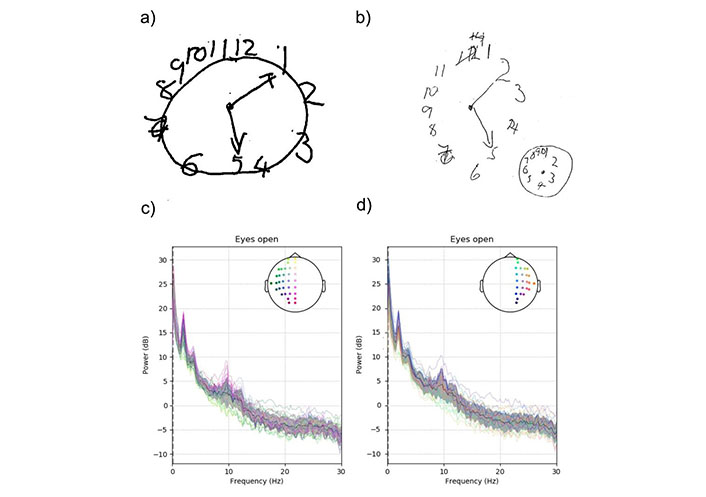
Cognitive and neurophysiological investigations. Clock drawing item: ACE-III 77/100 in 2020 (a); mini-ACE 20/30 one year later (2021) (b); power spectrum analysis of the whole brain is shown for the left hemisphere in (c) and the right hemisphere in (d). Each color-coded line represents an electrode. The black line and the grey area are the mean and standard deviation, respectively. An enhancement in slow waves, indicated by the peak at 2 Hz, was observed in both hemispheres. This was most pronounced in temporal and parietal electrodes. dB: decibel
After 3 months, the patient reported some improvement in memory being able to follow an entire recipe, and mentioned an improvement in ‘(brain) fog’. In addition, there was an improvement in his identification of right and left and his thought processes appeared clearer. Eleven months after the National Health Service (NHS) diagnosis, the patient and his wife reported ‘good and bad days’. with a discernible difference in his memory when he had the HBOT. A repeat FDG-PET scan again showed persisting reduced cortical metabolism predominates the parietal temporal distribution, with some improved cortical metabolism (reduced severity and extent) particularly at the medial and lateral occipital lobes. The repeated mini-ACE was 20/30 (Figure 1b). His repeated EEG, performed in April 2022, during quite wakefulness, revealed excess fronto-temporal and parietal slow wave activity (2 Hz), bilaterally (Figure 1c and d). The latest Rowland Universal Dementia Assessment Scale (RUDAS) cognitive test done in May 2023 was 28/30. He remains independent in activities of daily living and continues to drive. He states that he knows how to perform tasks, but (his) brain is not connected to (his) hands. Our patient continues with three times weekly HBOT sessions, and regular 10 mg donepezil mane. So far, he had about 400 sessions and counting.
All HBOT sessions have been held at the same hyperbaric centre, in a multiplace chamber accommodating up to 5 people, with each of them having oxygen flow through their own oxygen mask. Each session consisted of breathing 100% oxygen by mask at 2 ATA, and usually was with a duration of 50–60 min, to allow for compression/decompression time at rates 1.0 m/min. Our patient reported only mild dizziness and mild nausea over the few initial sessions. These side effects were transient and did not re-occur afterward in the course of repeated HBOT sessions.
This case report adds further information and support about HBOT use in aiding the treatment of AD. Our patient’s cognitive performance remained stable long-term, with worsening in the cognitive performance reported when not using the HBOT. The visuospatial performance improvement corresponded to the FDG-PET improved glucose metabolism in the parieto-occipital lobes. These findings are consistent with previous reports on the HBOT in both AD and amnestic MCI patients, with improved cognitive function, enhanced spatial and working memory, and increased brain metabolism [21, 29], albeit over a short period of time following HBOT. In healthy adults, HBOT similarly improved cognitive performance and spatial working memory, but not short- and long-term and immediate memory or attention-related cognitive measures [33]. In another study [18], it was the improvement in attention, executive function, and information processing speed and cognitive faculties that decline with ageing. Furthermore, this was associated with regional changes in cerebral blood flow, affecting predominantly the areas of the frontal lobe (corresponding to Broadman areas 6, 8–10) in the right hemisphere. In a detailed self-report on HBOT administered due to subjective memory problems in an 81-years-old man with a previous medical history of coronary artery disease with a history of a coronary artery bypass graft and a coronary stent [34], after 3 months of use, there was a rather modest improvement in a range of neuropsychological functions (3.1–3.8%), with a substantial improvement in the delayed verbal recall (27.1%). This was accompanied by improved perfusion on MRI perfusion brain scans (43.3–52.2%) in brain areas involved in memory, coordination, and vision, whereas on single photon emission CT (SPECT) brain scan the increased perfusion ranged from 8.79% to 16.12% in the temporal pole and entorhinal cortex. Thus, the HBOT observed improvement in neurocognition is further supported by neuroimaging modalities measuring cerebral perfusion and blood flow.
Earlier administration of hyperbaric oxygen post-injury, younger age at both the time of injury and hyperbaric oxygen administration, and a higher number of hyperbaric oxygen administrations are associated with improved outcomes [35]. This was the case with our patient, with the HBOT being started at an earlier age and in the earlier course of his cognitive deterioration, alongside the recommended antidementia treatment, and was maintained with prolong-term and regular HBOT intervention over a period of several years. A previous study reported a sustained HBOT benefit of one month in AD, and up to 6 months in amnestic MCI following only one course of HBOT [36]. Interestingly, prolonged HBOT sessions (43 in total) in an older patient with more advanced AD stage who was not on antidementia drugs but ‘treated’ with 6 CT brain scan sessions did not show these benefits [37].
The HBOT mechanism underlying the benefits in vascular and neurodegenerative dementias remains unknown. Possible mechanisms include restoration in brain blood supply and increased neurogenesis in animal models of vascular dementia [23], whereas transgenic AD animal models, only after 14 days of HBOT exposure, had attenuated neuroinflammatory processes and phosphorylated tau protein, accompanied by higher clearance of amyloid beta protein [33]. Hachmo et al. (2020) [38] reported that HBOT may induce significant senolytic effects including significant telomere elongation. Recent studies implicate humanin, a mitochondrial protein, that increases following HBOT, and this increase appears to be significantly correlated with cognitive improvement in people with vascular dementia [19]. These findings suggest that the HBOT has a direct influence on the AD core neuropathological hallmarks, i.e. neurofibrillary and amyloid pathology, cerebral blood flow, and inflammation, all closely correlated with the dementia clinical symptoms [39].
The heterogeneity of reported clinical assessments and outcomes in case reports [29, 34, 37] and small studies [19, 36], outcome measures and time recorded as well as the variability of reported HBOT protocols [40], makes it difficult to comment about the HBOT therapeutic impact in AD. The studies to date have indicated that one course of HBOT only temporarily ameliorates cognitive impairment in AD patients [36] and the effects may not be permanent. Furthermore, only one aspect of the AD clinical symptoms, cognitive performance, seems to have been addressed, but not the accompanying neuropsychiatric changes and/or quality of life. Thus, there need to be more robust and controlled clinical trials on a larger number of AD patients, with better quality of data and more in-depth analysis to determine the most effective treatment HBOT protocols that also address their impact on both cognitive and non-cognitive AD symptoms, its therapeutic efficacy on its own or as an adjuvant intervention to recommended antidementia therapies, and possible role in maintenance of achieved dementia clinical stability as well as duration of clinical benefits. In this context, the potential additional benefits of attending HBOT should not be overlooked. These may include providing a structure of the day and social contacts outside the family ties, alleviating caregivers’ burden, etc., aspects that have not yet been taken into account in the evaluation of HBOT benefits in dementia care.
HBOT has yet to find its place in the treatment of dementia. This may be due to the HBOT therapeutic support coming from observational studies, and, therefore, there is a lack of evidence-based practice that requires decisions about health care to be based on the best available, current valid, and relevant evidence. Since these decisions should be made by those receiving care, informed by knowledge of care providers and within the context of available resources, randomized control trials (RCTs) and/or systematic reviews on RCTs still provide the golden standard of evidence to help physicians make better and more informed decisions. One of the first RCT studies in the field [41] did not report the method of vascular dementia patients’ randomisation but reported that HBOT combined with donepezil could significantly improve cognition in these patients. Most recently, HBOT RCTs and their protocols have started emerging, with successful approaches in blinding both patients and researchers. Thus, the recently completed HBOT RCT in 73 randomized post-COVID patients demonstrated a significant improvement in brain MRI perfusion and microstructural changes in several cortical areas, indicating that HBOT can induce neuroplasticity and improve cognitive, psychiatric, fatigue, sleep, and pain symptoms of patients suffering from post-COVID-19 condition [42]. These beneficial effects may be due to HBOT increasing brain perfusion and neuroplasticity in brain areas associated with cognitive and accompanying behavioral and emotional roles that are also affected in dementia. The ongoing HBOT RCT protocol for MCI in people with type 2 diabetes mellitus involves 154 patients, with randomization done in clusters of 12 patients [43], with the primary outcomes being executive cognitive function and episodic memory. All participants will have a 3 Tesla (3T) MRI brain scan to evaluate their cerebral blood flow. Another ongoing RCT, with expected completion in spring 2025 year [44] will have 100 participants, with randomization being done in clusters of 6 patients due to the space in the multispace HBOT chamber. The three study technicians, who will activate the HBOT/sham protocol during the session times, will be the only unblinded staff. The neurocognitive and neuroradiological outcomes, although do not appear to have wider use for routine clinical settings since the neurocognitive battery and the PET-amyloid brain scans are not widely used for the majority of dementia patients, promise to provide the necessary answer to the usefulness of this therapeutic approach in augmenting the currently available antidementia treatments, or prevent the cognitive impairment progression to overt dementia. However, both HBOT RCT protocols for dementia use prolonged exposure to oxygen (90 min at 2 ATA), which may be difficult to compare to the previously conducted studies in the field.
In the current study, our patient initially used HBOT alone, and only later, when diagnosed with dementia by the NHS Memory Service, its use was augmented with the recommended antidementia therapy, donepezil, a cholinesterase inhibitor. Since to date, there is no cure for dementia, it is of interest to speculate about the role of HBOT in relation to novel antidementia drugs, especially their delivery along the nanotechnology medical advances. Thus, the latest advances in AD therapeutics [45, 46] may provide a further venue for the HBOT use in therapeutic purposes in dementia. Namely, besides the most commonly experienced side effects across the spectrum of the currently approved immunotherapies, such as immune-related adverse events (i.e. gastrointestinal, endocrine, and dermatologic adverse effects), the side effects of donanemab and lecanemab-irmb (both recommended for use in people with MCI or mild AD stages) are headaches, infusion-related reactions, and amyloid-related imaging abnormalities (ARIA), a side effect more pronounced in homozygous APOE ε4 carriers [47]. ARIA present with transient brain swelling and even life-threatening brain oedema and intracerebral haemorrhages, with clinical symptoms of headache, confusion, dizziness, vision changes, and nausea, as well as seizures. Due to some of these side effects, HBOT may find, therefore, its place in their prevention or be co-administered with these amyloid modifiable drugs. Namely, HBOT, by increasing oxygen tension and raising the oxygen brain tissue levels, can help decrease the intracranial pressure and relieve cerebral oedema [24] caused by these drugs. However, these HBOT benefits need to be explored further in prospective studies to determine its therapeutic efficacy.
HBOT has been previously described to facilitate the focused permeability and retention effect of nanoparticles and, thus, help to improve drug concentration in organs that are not so easily accessible, such as the brain glioma [48]. HBOT therapy has already been suggested to be a strategy to boost the therapeutic efficacy of nanomedicine against hypoxic solid malignancies [49]. This property can be used in other diseases, such as AD, targeting the molecular hallmarks of the disease, neurofibrillary (tau protein) tangles, and amyloid plaques. The integration of novel AD therapeutics with nanoparticles has the advantage of improving their ability to permeate the blood brain barrier. Furthermore, whilst improving the drug concentration in targeting AD hallmarks, the use of nanoparticle delivered drugs spares the non-affected tissue, resulting not only in enhanced antidementia effect but also in reduced adverse effects, which seem to be much more pronounced with the latest approved AD generation of therapeutics.
We have to bear in mind that the conventional administration route of the drugs (orally or intravenously, as is the case with the latest modifiable AD drugs), even when coupled with nanoparticles, may be somewhat limited, leaving up to 95% of the drug not delivered to its target(s). Similarly, remternetug, the third AD modifiable immunotherapeutics, pursues another route of administration (subcutaneous application) that faces the same issues as the intravenous delivery of drugs. Namely, intranasal administration of drugs has been shown to be more efficient in their delivery directly to the central nervous system within minutes along the olfactory and trigeminal nerve pathways, bypassing the blood-brain barriers [50], thus bringing the clinical utilisation of the nanomedicine closer to clinical setting.
Although the HBOT dementia therapeutic use seems to be hampered by both technical and methodological issues, this therapeutic strand offers numerous opportunities to be embedded in the first-line therapeutic management of dementia, which is not restricted to HBOT alone, but also augments the currently available antidementia drugs. Its role in prevention and treatment of the major side effects of the novel amyloid modifiable drugs as well as its potential in nanomedicine, i.e. novel mitochondria targeting agents [51], is the guarantee that seems to be closer to solving the major issues in delivering the novel antidementia treatments that are target the specific molecular substrates of various dementia subtypes, whilst sparing the unaffected brain tissue and minimising the drugs’ side effects. RCT clinical studies are now needed to bring HBOT closer to its clinical utility. Furthermore, purpose in life has been shown to decline with the emergence of cognitive impairment [52]. The intervention, such as HBOT prior to or following cognitive impairment may help maintain well-being in this population. This may also help further facilitate the development of the person-centred approach to dementia treatment and care.
ACE: Addenbrooke’s Cognitive Assessment
AD: Alzheimer’s disease
ATA: atmospheres absolute
CT: computerised tomography
FDG: fluorodeoxyglucose
HBOT: hyperbaric oxygen therapy
IL: interleukin
MCI: mild cognitive impairment
MRI: magnetic resonance imaging
PET: positron emission tomography
RCT: randomized control trial
We would like to acknowledge our patient’s and his family’s help with the case report. The patient and his next of kin provided an informed consent for publication of information contained in this case report. They were involved in various stages of the manuscript’s preparation and approved the final version of the manuscript. Most recently our patient started participating in the ‘Singing for the Brain’ project that entails choir singing, and plans to learn to play an instrument (ukulele). He continues to have regular follow-up with the Memory Service.
EBML: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Supervision. JS and MC: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. HJY, MS, and QA: Investigation, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
This study was approved by Integrated Research Application System (IRAS) ethical approval (IRAS ID 279309) and complies with the Declaration of Helsinki.
Informed consent was obtained from the patient.
Informed consent was obtained from the patient and his family.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
