Affiliation:
1National Research Council-Institute for Systems Analysis and Computer Science “Antonio Ruberti”, Via dei Taurini 19, 00185 Rome, Italy
2IRCCS Fondazione Santa Lucia, Via Del Fosso di Fiorano 65, 00143 Rome, Italy
Email: cinzia.volonte@cnr.it
ORCID: https://orcid.org/0000-0001-7362-8307
Explor Neuroprot Ther. 2023;3:1–7 DOI: https://doi.org/10.37349/ent.2023.00034
Received: September 08, 2022 Accepted: January 11, 2023 Published: February 21, 2023
Academic Editor: Abdelhamid Benazzouz, Bordeaux University, France; Rafael Franco, Universidad de Barcelona, Spain
More than 600 different neurological diseases affect the human population. Some of these are genetic and can emerge even before birth, and some are caused by defects, infections, trauma, degeneration, inflammation, and cancer. However, they all share disabilities caused by damage to the nervous system. In the last decades, the burden of almost all neurological disorders has increased in terms of absolute incidence, prevalence, and mortality, largely due to the population’s growth and aging. This represents a dangerous trend and should become our priority for the future. But what new goals are we going to set and reach now, and how will we exploit thought-provoking technological skills for making these goals feasible? Machine learning can be at the root of the problem. Indeed, most recently, there has been a push towards medical data analysis by machine learning, and a great improvement in the training capabilities particularly of artificial deep neural networks (DNNs) inspired by the biological neural networks characterizing the human brain. This has generated competitive results for applications such as biomolecular target and protein structure prediction, structure-based rational drug design, and repurposing, all exerting a major impact on neuroscience and human well-being. By approaching early risks for diseases, non-invasive diagnosis, personalized treatment assessment, drug discovery, and automated science, the machine learning arena has thus the potential of becoming the new frontier for empowering neuroscience research and clinical practice in the years ahead.
More than 600 different neurological diseases affect the human population according to the US National Library of Medicine. Some of these are genetic and can occur even before birth, and some are caused by defects, infections, trauma, degeneration, inflammation, and cancer, but they all share disabilities induced by injury to the nervous system. These diseases have globally represented the second leading cause of death, and the first leading cause of disability-adjusted life-years [(DALYs), i.e., the sum of years of life lost and years lived with disability] in 2016 [1]. Moreover, in the US from 1990–2017 the burden of almost all neurological disorders has further increased in terms of absolute incidence, prevalence, mortality, and DALYs estimates, largely due to the population’s growth and aging [2]. Although primary prevention of neurological disorders is now beginning to show a positive influence (perhaps due to improved quality and comprehensiveness of prevention strategies, enhanced education levels, and more general access to health care), the prevalence and impact of these disorders will further increase as health and living standards still improve and populations become older. This trend surely represents a dangerous warning and should become our priority for future healthcare planning, research resource allocation, and the benefit of the entire human population.
Most of the human genome is expressed in the brain, and neural circuits along with brain areas are highly intricate and interconnected. Thus, neurological diseases manifest in a wider array of symptoms and are vastly more complex than other domains of medicine. As a result, today we need to develop further tools that are proportionately capable of handling this huge degree of complexity and amount of information if we want to untangle these diseases.
Over the past decade, several milestone studies have pioneered cutting-edge means for neurological disease prevention, biomarker identification, diagnostic criteria, preclinical modeling, neuroimaging technologies, and network/systems medicine approaches. Today, the work of experts in different experimental fields aims to further improve molecular tools, instruments, and computational frameworks for inspecting the brain at multiple spatial and temporal scales. The new frontier has now to challenge once more our current thinking about neurological research, practice, and policy. We should now ask: what new goals are we setting and trying to reach, and how will we exploit thought-provoking technological skills for making these goals feasible?
An algorithm is a computational approach to solving a problem, and machine learning offers many different algorithms to solve a wide variety of problems. The first procedures of machine learning go back to the 1950s and by then the use of machine learning has increased dramatically [3]. The definition of machine learning by a pioneer and leader in the field, Mitchell [4], in his seminal book “Machine learning”, is that “a computer program is said to learn from experience E with respect to some task T and some performance measure P, if its performance on T, as measured by P, improves with experience E. A program uses machine learning if it continuously improves at problem solving with experience”. In other words, machine learning was introduced as a new approach to solving problems by designing algorithms that could learn spontaneously from data, evolve and autonomously improve their ability to make predictions through experience [5]. As such, machine learning can be considered an exciting tool and promising option for hopefully improving our skills also in neurological disease prevention, diagnosis, and treatment. Some of the most common and useful algorithms and approaches used in machine learning today are “artificial neural networks” and “deep learning”, but we should keep in mind that very often these approaches are used together, or interchangeably, to solve a given problem.
An artificial neural network is a computational model initially inspired by the biological neural networks characterizing the human brain. It uses a series of algorithms to process and translate an input signal into an expected output over several stages. This method is often used for instance in image recognition and language translation. Deep learning then refers to a family of machine learning algorithms that make heavy (deep) use of artificial neural networks, where “deep” refers to the vast number of layers, or iterations, between input and output (Table 1) [6]. As computing power is becoming less expensive, the learning algorithms in today’s applications are becoming always “deeper”. The huge flood of data we are experiencing nowadays inevitably calls for automated data analysis, which is exactly what machine learning provides in the form of algorithms that can detect patterns in big data, and then use the uncovered patterns to predict future data and perform further kinds of decision making under uncertainty. With the specific goal of solving tasks that are easy for people to perform and solve intuitively, but for which it is hard to formally describe how to obtain solutions, deep learning has further evolved and scaled up into a promising new cluster of machine learning algorithms defined as deep neural networks (DNNs) [7]. Despite the number of units and connections possessed by DNNs are several orders of magnitude less than the numbers of neurons and synapses in the human brain (estimated to be 100 billion neurons transmitting signals to each other via as many as 1,000 trillion synapses [8]) these artificial networks can perform many tasks, at a level comparable to that of humans and sometimes beyond while being able to process a much larger amount of data in a much smaller fraction of time [9].
Machine learning versus deep learning
| Features | Machine learning | Deep learning |
|---|---|---|
| Data setting | Structured | Not structured |
| Learning | By operator | Intrinsic |
| Database | < 1 × 108 data points | > 1 × 108 data points |
| Algorithm | Variable | Artificial neural network |
| Problem solving | By partitioning | End-to-end approach |
| Execution time | Long model test time | Short model test time |
| Application field | Routine operations | Complex systems |
| Hardware | Low-end machine | High-end machine |
| Suitability | Simple problems | Complex problems |
Unlike software algorithms requiring precise instructions, DNNs can autonomously learn rich hierarchical feature representations, by summarizing the most relevant information necessary to achieve a specified task on a large corpus of data. Furthermore, they can increasingly improve their proficiency by adopting the quantitative approach to observing progressively more examples as input. In the last decade, driven by significant advances in both computer hardware and available data, there has been a great improvement in the training capabilities of DNNs, which have been successfully applied for instance to image and speech recognition. Most recently, there has been even a further push towards DNNs medical data analysis, to the point of generating competitive results for applications such as biomolecular target and protein structure predictions, structure-based rational drug design, drug repurposing, all exerting major impacts on human well-being and health care [10, 11].
At present, when we approach for instance neurological disorders through machine learning, we can deal with the following main scenarios:
(i) Early risk assessment: it means that the ability of deep learning to find patterns of correlations when looking at the vast genome database [12] and unveiling new connections between disparate parts of the human genome and for instance neurological diseases [13], can be successfully exploited for the early assessment of disease risk. Clearly, this raises an alarm for identifying pre-symptomatic people at risk and putting them on preventative plans. As a further step of this process, deep learning can be adopted to create, improve, and maintain a health portrait for each person, by leveraging genome sequencing data together with bio-imaging and clinical laboratory testing, always with the aim to better personalize neurological disease risk assessment, and give early warnings and most proper recommendations to prevent deranged behaviors.
(ii) Non-invasive diagnosis and patient stratification: we know that phenotypes and individual parameters such as face morphometry, voice tones, musculoskeletal structure and morphology, sensorimotor coherence, and brain activity distinguish individual anatomy and underlying pathophysiology. They are the foundations of predictive modeling applied to non-invasive diagnosis, patient stratification, and delivery of tailored treatments [14, 15]. After scanning unconventional and progressive derangement of these parameters [for instance by advanced three-dimensional (3D) video sensors systems such as Kinect and Intel RealSense, synchronized with voice recordings, 3D magnetic resonance imaging data, and electroencephalogram plots], deep learning algorithms can indeed recognize, extract and index quantitative non-invasive information related to phenotypic variabilities and pathological decline associated to disease insurgence, progression, or aging.
(iii) Personalized treatment evaluation: by implementing the above-described features, deep learning can thus prioritize pathological hallmarks and big data from patients for monitoring purposes, precision medicine, and therapeutic benefit over time [16]. This will allow us not only to track, but also follow up on the insurgence, progression, and personalized treatment not of a single neurological disorder, but of any general state perturbing our standardized and age-correlated morphological, vocal, motion, and neural activity features. Emerging computational improvements obtained in genome-informed counseling will also allow us to predict which patients are likely to need medication for which genomic information. As such, artificial intelligence will soon become omnipresent across the entire panorama of health care, by showing unprecedented opportunities.
(iv) Drug discovery: predictive modeling by machine learning is estimated to play a key role in accelerating and further improving also the long “iter” of drug discovery. For instance, “promiscuous” molecules with multi-target activities are the goal of poly-pharmacology, a renewed approach, especially against non-cell autonomous, heterogeneous, and multifactorial neurological diseases that distinguish the nervous system. Indeed, machine learning is crucial for developing novel drug design strategies, by systematically discriminating for instance multi-target from single-target compounds and rationalizing and hierarchical ordering their activities within a methodological computational framework. Moreover, machine learning finds key applications in drug repurposing as well, by speeding up the pre-clinical design of novel drugs, and saving times and costs compared to the more traditional ex novo drug discovery process [17]. Drug repurposing is already supported by network-based approaches applied to massive observed data from neurological diseases and existing drugs [18–21]. The tremendous growth of both publicly available machine learning methods and pre-clinical/clinical large-scale science data thus finds its up-to-date and prominent application not only in monitoring diseases but also identifying new drug targets or novel uses of already marketed therapeutics, with the least error.
(v) Automating science: going even forward, techniques from artificial intelligence can be implemented to perform a series of automated scientific experiments, in other words, to automate science by robot scientists. This represents a powerful way not only for implementing scientific procedures but also for bringing research to higher superhuman performance and efficiency levels. Indeed, automated science formulates a series of hypotheses around a scientific question, selects the most proficient experiments to be performed for discriminating among the formulated hypotheses, runs experiments using laboratory automation equipment, and finally analyzes the results. For instance, the robot scientist “Eve” has found applications from early-stage drug development to semi-automated reproducibility testing in cancer biology [22], and “Genesis” can run up to 10,000 cycles of hypothesis-led experiments in parallel per day, showing scientific skills that are not substituting, but complementary, to human scientists.
A very fruitful research about machine learning has resulted so far in many new algorithms being developed, and a variety of new uses. For instance, companies in the biomedical sector are taking advantage of the overwhelming power of machine learning, and medical research organizations are using machine learning to analyze enormous amounts of human health data, in an attempt to identify patterns in disease conditions and improve the management of neurological diseases and healthcare. Machine learning can indeed be applied to investigate for instance risk factors, and predict complications in treatments used for neurological disorders. Some major limitations, however, still consist in understanding the machine learning model when performing the decision-making process, or in the limited biological data size to be supplied to machine learning processing. However, this last issue has started to be overcome by the availability of public datasets. It is worth mentioning for this purpose, the Human Connectome Project (http://www.humanconnectomeproject.org/) and the Alzheimer’s Disease Neuroimaging Initiative (ADNI, http://adni.loni.usc.edu/). As such, the machine learning arena has undoubtedly the potential of becoming the new frontier for empowering neurological research and advancing public health (Figure 1). By approaching early disease risk, non-invasive diagnosis, personalized treatment assessment, drug discovery, and automated science, machine learning will be at the forefront of basic research and clinical practice in the years ahead [23], because of its increased capability of finding patterns in large amounts of data, collecting lots of genomic information, emitting predictions that are physiologically or pathologically relevant, and its applicability to images, audio, motion sensors, neural oscillations, genetic/drug data, and laboratory testing.
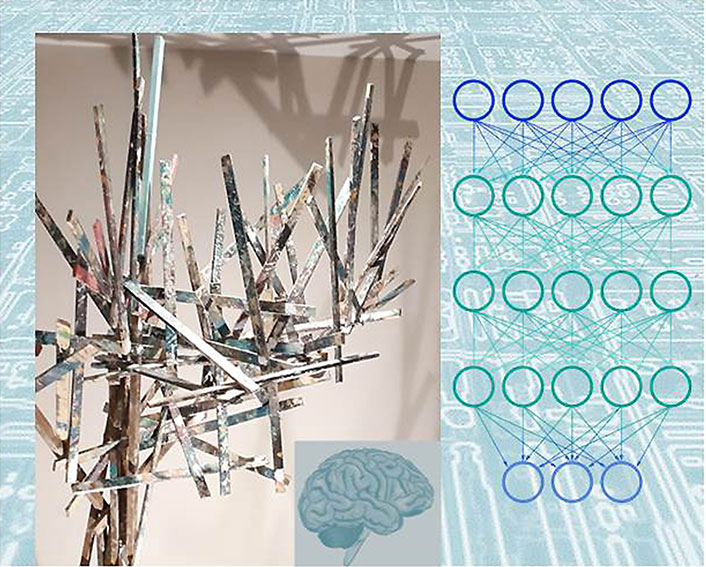
From brain convolutions to circuits through artificial neural networks. The brain is a complex neural network. The colored bars in the center brownish panel exemplify different architectures of algorithms (schematized on the right) at the interface of brain convolutions (small inset) and circuits (background light blue panel). Algorithms in artificial neural networks can be exploited for empowering neuroscience research and clinical practice in the years to come
Thus, our expectations from now on will be: i) to leverage machine learning algorithms for assisting people through all facets of neurological disease prevention, diagnosis, and treatment, in a non-invasive and most importantly, personalized way; ii) to “open source” the research center quality care for neurological diseases, by bringing it right into the pocket of each person. Machine learning is surely at the root of this challenge.
DNNs: deep neural networks
I thank Dr. Matteo R. Ronchi (Caltech, Pasadena, USA) and Dr. Joseph L. Marino (DeepMind, London, UK) for inspiring this work, critically reading the text, and, most of all, for providing insightful discussions that deepened and broadened my understanding of machine learning.
The author contributed solely to the work.
The author declares that she has no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
The author’s research is supported by FATALSDRUG Project [Progetti di Ricerca@CNR SAC.AD002.173.058] from National Research Council, Italy. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 5898
Download: 68
Times Cited: 0
