Affiliation:
1Department of Psychiatry, PC Sint-Amandus, 8730 Beernem, Belgium
Email: wostyn.peter@skynet.be
ORCID: https://orcid.org/0000-0001-7726-9751
Explor Neuroprot Ther. 2022;2:110–117 DOI: https://doi.org/10.37349/ent.2022.00022
Received: March 13, 2022 Accepted: May 06, 2022 Published: June 20, 2022
Academic Editor: Abdelhamid Benazzouz, Bordeaux University, France
The glymphatic system, first described in 2012, is a brain-wide perivascular network that plays an important role in promoting interstitial metabolic waste removal from the brain. Glymphatic pathway function has been reported to be dramatically diminished in the aging brain. Furthermore, glymphatic system dysfunction has been linked to a spectrum of neurodegenerative diseases including Alzheimer’s disease (AD). This waste clearance pathway of the brain is most active during sleep and is largely disengaged during wakefulness. While norepinephrine (NE) is responsible for suppressing the glymphatic function, electroencephalographic slow-wave (delta) activity has a facilitating effect. An intriguing question is whether these regulators of glymphatic activity can be modulated by meditation-based approaches and whether such approaches have the ability to increase glymphatic function in the awake brain. The present article hypothesizes that meditation-based approaches, such as immersive sound meditation, may have the potential to enhance glymphatic pathway transport and solute clearance by reducing NE and increasing slow-wave activity. If confirmed, meditation could be an attractive approach to promoting healthy brain aging and to preventing neurodegenerative conditions like AD.
The lymphatic circulation extends throughout most of the body and plays a critical role in tissue homeostasis and function by facilitating the clearance of extracellular proteins and excess fluid from the interstitium [1, 2]. However, the brain and central nervous system lack a conventional lymphatic drainage system [1]. This has led to long-standing questions about how interstitial fluid (ISF) in the brain is cleared of waste, especially given that the high metabolic activity of the brain requires an efficient system for the elimination of metabolic waste products [1, 2]. Furthermore, several neurodegenerative diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease, are characterized by the accumulation of misfolded proteins that aggregate and trigger neurotoxicity [3]. One example of such a protein is amyloid-β (Aβ). It accumulates in the brain during normal aging and its accumulation is one of the hallmarks of AD pathogenesis. A decreased effectiveness in the clearance of misfolded proteins plays a critical role in their build-up and spread throughout the brain [3]. Importantly, recent discoveries have shed light on the enigma of brain waste clearance [2, 4, 5]. The “glymphatic system” is a newly discovered brain-wide clearance system that subserves a lymphatic-like function and that participates in the removal of Aβ from the brain [4]. More recently, two independent studies reported the presence of a network of traditional lymphatic vessels in the dura mater of the mouse brain [2, 5]. These studies were in line with the work of the Italian anatomist Paulo Mascagni who in 1787 was the first to describe lymphatics in the human dura mater in his masterpiece “Vasorum lymphaticorum corporis humani historia et ichnographia” [6]. These studies further suggested a connection between the newly identified meningeal lymphatic vessels and the glymphatic system. It was suggested that dural lymphatic vessels absorb cerebrospinal fluid (CSF) from the adjacent subarachnoid space and brain ISF via the glymphatic system, thus acting as a CSF outflow route while contributing to immune surveillance of the central nervous system [2].
An increasing body of evidence suggests that malfunction of the glymphatic-lymphatic fluid transport system may underlie neurodegenerative disorders involving the accumulation of misfolded proteins, such as AD [7, 8]. As such, targeting the glymphatic-lymphatic system could provide new therapeutic avenues for neurodegenerative proteinopathies. Discovering strategies for modulating glymphatic and meningeal lymphatic functions could result in the delay of cognitive decline and the prevention of neurodegenerative disorders. Here, we speculate that meditation-based approaches may increase glymphatic function, thereby facilitating efficient brain waste clearance, further promoting healthy brain aging, and reducing the individual risk of developing neurodegenerative diseases such as AD.
In 2012, the “glymphatic system” was first described and characterized in mice by a team of researchers headed by Iliff and Nedergaard [4] as a brain-wide perivascular clearance pathway. This glial-dependent system for CSF-ISF exchange plays an important role in the elimination of brain metabolic waste products, including Aβ [4]. Astrocytes play a key role in the glymphatic pathway. Astrocytes create with their vascular endfeet an outer wall around the cerebral vasculature (Figure 1) [9, 10]. This arrangement creates the perivascular spaces that differ from the rest of the neuropil by its low resistance to fluid flow. The perivascular spaces allow fast transport of CSF into deep brain regions. CSF flows into the brain parenchyma from periarterial channels, and exchanges with ISF in a process facilitated by aquaporin-4 (AQP4) water channels abundantly expressed on astrocytic endfeet (Figure 1) [4, 9, 10]. The CSF/ISF mixture subsequently exits the brain parenchyma along perivenous pathways from where it travels to the cervical lymphatic system (Figure 1) [4, 9, 10]. The combined effects of arterial pulsatility, respiration, slow vasomotion, and CSF pressure gradients play a role in driving CSF from the subarachnoid space into the Virchow-Robin perivascular spaces [10]. This perivascular network was called the “glymphatic system” after its dependence on astroglial water flux and its lymphatic-like function. While the glymphatic system is considered to play a major role in the removal of metabolic waste from the brain, this pathway also contributes to the delivery of nutrients, such as glucose, amino acids, lipids, and neuromodulators, throughout the brain [10]. Glymphatic pathway function has been reported to be dramatically diminished in the aging brain [11]. In the aging rodent brain, a reduction in cerebral arterial wall pulsatility and widespread loss of perivascular AQP4 polarization along the penetrating arteries accompanied the decline in CSF-ISF exchange [11]. Studies have also demonstrated that the glymphatic system is impaired in AD animal models and in patients with AD [7, 8].
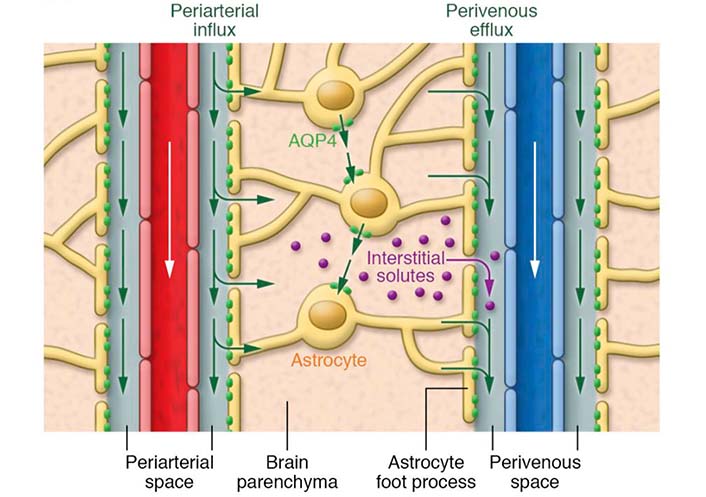
Schematic outline of the brain glymphatic pathway. CSF flows into the brain parenchyma from periarterial channels, and exchanges with ISF in a process facilitated by AQP4 water channels. These channels are densely present at the astrocytic vascular endfeet that create an outer wall around the cerebral vasculature. The CSF/ISF mixture subsequently exits the brain parenchyma along perivenous pathways. The blood flow direction is indicated with white arrows. Green arrows indicate the movement of CSF and ISF. Purple dots indicate interstitial solutes [9]
Note. Adapted from “Understanding the functions and relationships of the glymphatic system and meningeal lymphatics,” by Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J. J Clin Invest. 2017;127:3210–9 (https://www.jci.org/articles/view/90603). © 2017 American Society for Clinical Investigation.
Understanding the mechanisms regulating the glymphatic system may help in the identification of health approaches and strategies that could promote healthy brain aging and reduce the risk of neurodegenerative disorders. An intriguing finding regarding the glymphatic system is that this pathway is primarily active during sleep and anesthesia, while largely disengaged during wakefulness [12]. It has been shown in mice that slow-wave sleep and anesthesia with ketamine/xylazine are associated with a 20-fold increase in glymphatic CSF influx and a doubling in glymphatic clearance of Aβ when compared to the awake state [12]. Furthermore, the interstitial volume fraction increased by more than 60% during natural sleep or ketamine/xylazine anesthesia compared with wakefulness, supporting the notion that CSF influx and interstitial solute efflux are suppressed during wakefulness as a result of contraction of the interstitial space which results in increased resistance to fluid transport [12].
The locus coeruleus, a small nucleus located in the brainstem, is the brain’s primary source of the catecholamine norepinephrine (NE) which serves as a neuromodulator. NE is increased after stressful stimuli and plays a prominent role in arousal [13]. Interestingly, in awake mice, it was shown that blockade of adrenergic signaling increased the interstitial volume fraction, enhanced glymphatic CSF influx, and was associated with slow-wave electrocorticography activity [12]. These findings suggested that changes in locus coeruleus-derived noradrenergic tone are involved in the modulation of not only the cortical neuronal activity but also the size of the interstitial space [12]. NE released during arousal leads to a contraction of the extracellular space volume fraction, increasing interstitial resistance and therefore reducing CSF influx and ISF efflux [12]. NE also inhibits the secretion of CSF from choroid plexus epithelial cells, further leading to the suppression of glymphatic function [10].
While NE is responsible for suppressing the glymphatic function, electroencephalogram (EEG) slow-wave activity (SWA) apparently has a facilitating effect [12]. Slow waves or delta waves, which are high amplitude brain waves with a frequency of 0.5–4 Hz, are related to deep sleep and are found during certain types of anesthesia [13]. These slow waves are a well-established indicator of sleep pressure [13]. Analysis of recordings from electrocorticography revealed that sleep- or ketamine/xylazine anesthesia-induced increases in glymphatic CSF influx and interstitial space volume fraction correlated with increased delta power (an increased quantity of delta wave recordings) compared to wakefulness [12].
Given the key role of SWA and noradrenergic tone in glymphatic function, an intriguing question is whether these regulators of glymphatic activity can be modulated by meditation-based approaches.
Four common meditation practices include focused attention meditation (FAM), open-monitoring meditation (OMM), transcendental meditation (TM), and loving-kindness meditation (LKM) [14]. During FAM, the practitioner has to focus attention on a chosen concept or object, such as breathing, physical sensation, or a visual image [14]. FAM includes Himalayan Yoga, Mantra, and Metta [14]. In contrast to FAM, OMM does not involve sustaining selective attention on a chosen object [14]. Instead, it is characterized by the monitoring of awareness itself, with no judgment and with acceptance of internal and external cues [14]. OMM includes Zen, Isha Yoga, Shoonya Yoga, and Vipassana [14]. During TM, the practitioner uses a mantra (i.e., a particular sound that has no literal meaning) to be aware of the present without an object of thought [14]. LKM practitioners focus on generating feelings of love and compassion first towards themselves, and then gradually extend this love to those one does not know, followed by those one dislikes [14].
With regard to the neuromolecular aspects of meditation, one would intuitively expect lower levels of plasma catecholamines in meditators, given that meditation can promote physiological responses opposite to stress responses [15]. Although this has not been a consistent finding [15], lower levels of NE have been found in meditation practitioners compared to control persons [16, 17]. A previous study compared NE blood levels between two groups of heart failure patients: a meditation group and a control group [16]. A significant decrease occurred in NE blood levels in the meditation group after meditating. No significant change occurred in NE blood levels in the control group. In another study, regular practitioners of either TM or Sidhi-TM techniques expressed significantly lower morning and evening plasma NE levels than the group of healthy controls [17]. However, it should be noted that lower NE blood levels do not necessarily reflect reduced central locus coeruleus NE activity.
A substantial number of EEG studies have investigated the neural oscillatory patterns during various types of meditation, including FAM, OMM, TM, and LKM [14]. In this regard, it has been demonstrated that there are distinct differences in neural oscillatory activity among these meditation practices [14]. Of particular interest here are the changes in delta oscillations during meditative states, given the facilitating effect of SWA on the glymphatic function. While a full review is beyond the scope of this paper, we briefly discuss evidence from several studies that demonstrate increased delta power across a variety of meditation practices. A study found that compared to controls, experienced (12.3 ± 5.6 years) Zen meditators had an increase in delta activity mainly in the medial prefrontal cortex during resting states [18]. Yet another study recorded EEG signals from Qigong meditators during resting, that were compared to a control group [19]. This study reported increased delta activity (and thus inhibition) in parts of the prefrontal cortex and anterior cingulate cortex in meditators, whereas other areas showed reduced delta activity (and thus stronger activation). A loving-kindness study used EEG to measure spectral changes in neural activity and demonstrated significant increases in delta frequency power associated with meditation compared to the baseline [20]. This study comparing the LKM practice to rest found that meditation was associated with significant increases in the delta frequency band over right temporal, right frontal, right occipital, and left and right parietal electrodes [20].
Considering the above, we hypothesize that meditation-based approaches could have beneficial effects on the glymphatic system by decreasing NE levels and enhancing slow-wave delta oscillations. Future research is needed to address several important questions. For example, what are the effects of meditation-based approaches on the glymphatic system, and what are the mechanisms through which these approaches may exert their effects? Can meditation increase glymphatic function in the awake brain? Can increased sleep pressure in wakefulness enhance glymphatic CSF influx [13]? Can occurrences of local delta power in particular parts of the awake brain locally modulate glymphatic solute transport and fluid fluxes [13]? If glymphatic clearance can be manipulated by meditation-based approaches, such approaches could have important preventive and therapeutic implications for brain health. Here, we propose immersive sound meditation as a candidate modulator of glymphatic function.
Since time immemorial, music and sound have been used for therapeutic and meditation purposes [21]. Tibetan singing bowls, for example, have been used for centuries by Tibetan monks in meditation practice [21, 22]. While sound meditation is not a new concept, studies exploring the potential physical and mental health benefits of this type of meditation are scarce [21, 22]. Immersive sound is a realistic, multi-dimensional sound experience where the listener is fully enveloped by sounds, which are produced over loudspeakers or headphones and are perceived as coming from an infinite number of points. It offers an experience exactly as it would be in real life, blurring the lines between digital and physical. Interestingly, immersive sound can also be used as a tool for building a regular meditation practice. Indeed, sounds can be specially created to suit meditation-specific characteristics. Such a meditation-based approach has the advantage of being a non-invasive and safe method. Furthermore, it is easy to apply and does not require significant discipline or a steep learning curve from the meditator. Compared to costly medications and other more traditional medical therapies, it is a relatively inexpensive approach where a person may choose to participate in group sessions.
As noted above, changes in the EEG delta activity have been reported in relation to meditation practices. Furthermore, different types of external stimuli, including auditory stimuli like music, can modify the bioelectrical activity of the brain [23]. A previous study investigated the effect of auditory stimulation on SWA [24]. Acoustic stimulation appeared to be especially effective in enhancing slow waves. It produced a diffuse increase in SWA across the scalp. Furthermore, the topography of enhanced slow waves was indistinguishable from the topographic profile of spontaneous slow waves observed during natural sleep. The authors concluded that acoustic stimulation appears to be a promising non-pharmacological means for inducing slow waves [24]. From this point of view, immersive sound meditation could be used to change brainwave activity, and especially to increase delta activity. Such immersive sound enhancement of slow-wave delta oscillations might be accompanied by a decrease in NE levels as has been reported in the context of meditation practices (at least in the blood) [16, 17]. Therefore, immersive sound meditation could serve as an attractive candidate modulator of glymphatic function. If confirmed, immersive sound meditation would represent an innovative approach for promoting healthy brain aging and preventing neurodegenerative disorders such as AD.
Understanding how meditation-based approaches modulate the noradrenergic tone and SWA may help elucidate the possible beneficial effects of meditation on the glymphatic function. In the present article, we propose that meditation-based approaches, such as immersive sound meditation, could be new promising approaches to delay cognitive decline and prevent neurodegenerative disorders by decreasing NE and inducing slow-wave delta oscillations, thereby promoting glymphatic clearance. Obviously, further research is needed to elucidate the potential effectiveness of meditation-based approaches in improving glymphatic function. If confirmed, such approaches might become increasingly important in daily practice for maintaining brain health across the human life span.
AD: Alzheimer’s disease
AQP4: aquaporin-4
Aβ: amyloid-β
CSF: cerebrospinal fluid
EEG: electroencephalogram
FAM: focused attention meditation
ISF: interstitial fluid
LKM: loving-kindness meditation
NE: norepinephrine
OMM: open-monitoring meditation
SWA: slow-wave activity
TM: transcendental meditation
PW wrote the first draft of the manuscript. PG wrote a section of the manuscript. All authors approved the final version of the manuscript.
Piet Goddaer has an ownership interest in the copyrights to his compositions. No conflicting relationship exists for Dr. Peter Wostyn.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
