Affiliation:
Department of Biotechnology, School of Applied Sciences, REVA University, Bengaluru 560064, Karnataka, India
ORCID: https://orcid.org/0000-0002-2404-3254
Affiliation:
Department of Biotechnology, School of Applied Sciences, REVA University, Bengaluru 560064, Karnataka, India
Email: senthilanal@yahoo.com
ORCID: https://orcid.org/0000-0002-8404-8209
Explor Neuroprot Ther. 2025;5:1004105 DOI: https://doi.org/10.37349/ent.2025.1004105
Received: February 12, 2025 Accepted: April 22, 2025 Published: May 12, 2025
Academic Editor: Raymond Chuen-Chung Chang, The University of Hong Kong, China
The article belongs to the special issue GPCR Heteroreceptor Complexes as Key Players in Neuroprotection
Neuroinflammation is a hallmark of various neurodegenerative and neuropsychiatric disorders, driven by complex interactions between neurotransmitter receptors and immune signaling pathways. Among these, heteroreceptor complexes—functional assemblies formed by the physical interaction of different G protein-coupled or ionotropic receptor subtypes within the same membrane microdomain—play a crucial role in modulating synaptic activity, neuroimmune responses, and inflammatory cascades. For example, the A2A-D2 receptor complex modulates dopaminergic signaling in the striatum and has been implicated in Parkinson’s disease pathology. These receptor-receptor interactions influence key signaling pathways involving dopamine, serotonin, glutamate, adenosine, and cannabinoid systems, thereby contributing to the pathophysiology of Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, schizophrenia, and depression. Dysregulation of heteroreceptor complexes disrupts neuronal homeostasis, exacerbates neuroinflammatory responses, and influences microglial and astrocytic activation. Understanding the molecular mechanisms governing these interactions, including allosteric modulation and biased agonism, offers novel therapeutic avenues for targeting neuroinflammation. Pharmacological strategies, such as selective allosteric modulators, biased agonists, and receptor-specific ligands, aim to restore heteroreceptor function and mitigate neuroinflammatory damage. Emerging clinical trials—such as those evaluating A2A receptor antagonists like istradefylline for Parkinson’s disease and 5-HT2A antagonists for schizophrenia—have shown promising neuroprotective and anti-inflammatory effects, although larger-scale, long-term studies are needed to confirm efficacy. This review highlights the pivotal role of heteroreceptor complexes in neuroinflammation, discusses their therapeutic potential, and underscores the need for further research into their functional dynamics to develop effective interventions for neurodegenerative and neuropsychiatric diseases.
Neuroinflammation is a complex and dynamic process that plays a crucial role in both neuroprotection and neurodegeneration. It is primarily mediated by glial cells, including microglia and astrocytes, in response to various stimuli such as infection, injury, or neurodegenerative diseases (Figure 1) [1]. While acute neuroinflammation serves as a protective mechanism by clearing pathogens and damaged cells, chronic neuroinflammation contributes to neuronal dysfunction and cell death, leading to the progression of neurodegenerative disorders like Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS). The sustained release of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1 beta (IL-1β) not only promotes gliosis but also initiates a cascade of harmful events by enhancing oxidative stress and disrupting neurotransmitter balance [2, 3]. This disruption can impair synaptic signaling and plasticity, further compromising neuronal communication. Additionally, activation of toll-like receptors (TLRs) and nuclear factor kappa B (NF-κB) signaling pathways amplifies inflammatory responses, creating a pathological loop that perpetuates neuronal damage. This self-reinforcing cycle contributes to neuronal death through mechanisms such as excitotoxicity, mitochondrial dysfunction, and impaired autophagy. Understanding the molecular underpinnings of neuroinflammation is essential for identifying novel therapeutic targets and developing effective interventions to mitigate its deleterious effects on brain function [4, 5]. Emerging strategies include the use of anti-inflammatory agents like minocycline, immune modulators targeting microglial activation, and neuroprotective compounds such as cannabinoids [6] and Nrf2 activators, which show promise in preclinical and early clinical studies [7, 8].
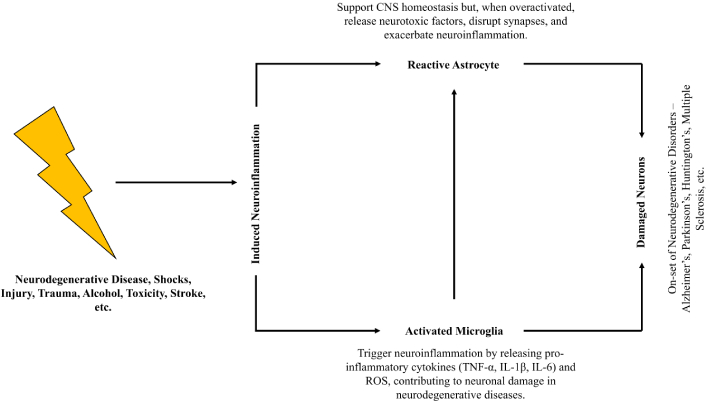
Generalized mechanism of induced neuroinflammation. CNS: central nervous system; TNF-α: tumor necrosis factor-alpha; IL-1β: interleukin-1 beta; IL-6: interleukin-6
Neurotransmitter receptors play a fundamental role in maintaining brain homeostasis by modulating synaptic transmission, neuronal excitability, and neuroplasticity [9]. These receptors, classified into ionotropic and metabotropic types, respond to key neurotransmitters such as glutamate, dopamine, serotonin, gamma-aminobutyric acid (GABA), and acetylcholine [10]. Excitatory neurotransmitters like glutamate activate N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, which are crucial for synaptic plasticity and cognitive functions. Conversely, inhibitory neurotransmitters like GABA bind to GABA_A and GABA_B receptors to regulate neuronal excitability and prevent excitotoxicity [11].
Neuroinflammation disrupts neurotransmitter receptor signaling, leading to synaptic dysfunction and neuronal loss. Pro-inflammatory cytokines can alter receptor expression, desensitize synaptic responses, and impair receptor trafficking [12]. For instance, increased levels of TNF-α have been shown to enhance AMPA receptor expression, exacerbating excitotoxicity in neurodegenerative conditions [13]. Similarly, dopaminergic dysfunction due to neuroinflammation is a key pathological feature in PD, where microglial activation and cytokine release lead to the progressive loss of dopaminergic neurons in the substantia nigra [14]. The interaction between inflammatory mediators and neurotransmitter receptors underscores the need to explore their crosstalk in the context of neurodegenerative diseases [15].
Heteroreceptor complexes are emerging as critical modulators of brain function, serving as integrators of multiple signaling pathways by facilitating receptor crosstalk [16]. These complexes are formed through the physical and functional interaction of different neurotransmitter receptors, altering their pharmacological and signaling properties. Notable examples include dopamine-glutamate receptor complexes (D2-NMDA, D1-NMDA), adenosine-dopamine (A2A-D2) [17], and serotonin-dopamine (5-HT2A-D2) receptor interactions [18, 19]. By modulating neurotransmission, synaptic plasticity, and neuroinflammatory responses, heteroreceptor complexes contribute to brain homeostasis and neuroprotection [20].
In the context of neuroinflammation, heteroreceptor complexes play a dual role by either exacerbating or mitigating inflammatory responses depending on their configuration and signaling dynamics. However, it is important to note that the existence and stability of these heteromers under pathological conditions remain an active area of investigation. In some disease states, receptor expression may be downregulated or altered, potentially preventing heteromer formation [21]. Further studies using post-mortem tissues, disease models, and advanced imaging techniques are needed to confirm whether these complexes are maintained or disrupted in patients with neuroinflammatory disorders. For instance, the A2A-D2 receptor complex is known to regulate dopaminergic neurotransmission, and its dysfunction has been implicated in neuroinflammatory processes observed in PD [22]. Conversely, activation of A2A receptors within these complexes can also suppress microglial activation and inhibit pro-inflammatory cytokine release, thereby exerting anti-inflammatory effects in certain neurodegenerative conditions. Similarly, alterations in the balance between excitatory and inhibitory receptor complexes contribute to synaptic dysfunction in neurodegenerative diseases [23]. Targeting heteroreceptor complexes offers a promising therapeutic avenue, as modulating their interactions can restore receptor function and attenuate neuroinflammatory damage [24]. Recent studies suggest that allosteric modulators and biased agonists can selectively influence heteroreceptor complex activity, paving the way for precision medicine approaches in neurodegenerative and psychiatric disorders [25]. For instance, several A2A receptor antagonists and 5-HT2A inverse agonists are currently being evaluated in preclinical and early-phase clinical trials for their potential to modulate neuroinflammatory pathways and improve cognitive or motor symptoms in PD and schizophrenia models.
Understanding the interplay between neuroinflammation and heteroreceptor complexes provides a new dimension in neuropharmacology and disease management [26]. Given their intricate role in regulating neurotransmission and neuroinflammation, further research into heteroreceptor complexes could unlock novel therapeutic strategies aimed at mitigating chronic neuroinflammatory conditions and improving neurodegenerative disease outcomes [27, 28].
This review was conducted to synthesize current knowledge on the role of heteroreceptor complexes in neuroinflammation and their therapeutic potential in neurodegenerative and neuropsychiatric disorders. Literature was searched across multiple databases, including PubMed, Scopus, and Web of Science, from January 2000 to March 2025. The search strategy employed Boolean operators and keywords such as “heteroreceptor complexes”, “neuroinflammation”, “dopamine receptors”, “glial activation”, “biased agonism”, and “allosteric modulators”. Articles were selected based on relevance, peer-reviewed status, and English language. Reviews, original research articles, and clinical trial reports were included. Studies focusing solely on monoreceptor mechanisms or non-CNS inflammatory models were excluded. Data extraction was performed independently by both authors to ensure consistency and reliability. A PRISMA-based flow diagram summarizing the selection process is included in Figure 2.
Heteroreceptor complexes are specialized molecular assemblies formed by the direct physical interaction of two or more different neurotransmitter receptors. Unlike monomeric receptors that function independently, heteroreceptor complexes allow for dynamic receptor crosstalk, leading to modified pharmacological and functional properties [16]. These complexes can form between receptors of the same family, such as different dopamine receptor subtypes, or between distinct neurotransmitter systems, like dopamine-glutamate or serotonin-dopamine interactions [29]. The presence of these receptor assemblies provides a sophisticated regulatory mechanism for synaptic transmission, neuroplasticity, and neuroinflammatory responses (Figure 3) [30].
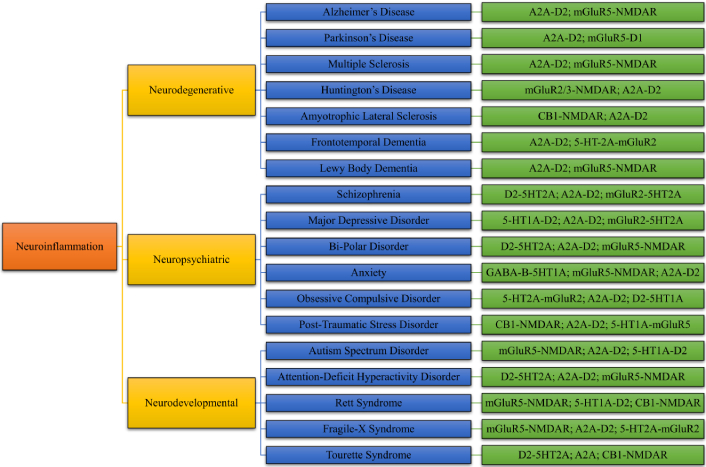
A diverse range of neurological disorders associated with neuroinflammation and their respective heteroreceptors
The classification of heteroreceptor complexes is based on their structural and functional properties. Homomeric complexes involve identical receptor subunits that enhance function through cooperative interactions [31]. In contrast, heteromeric complexes consist of different receptor subtypes, integrating multiple signaling pathways to regulate neurotransmission. Some heteroreceptor complexes are transient, existing only under specific physiological conditions, whereas others form stable interactions, contributing to synaptic regulation and neuronal function [32]. Several well-characterized heteroreceptor complexes, including dopamine-glutamate (D1-NMDA, D2-NMDA), adenosine-dopamine (A2A-D2), and serotonin-dopamine (5-HT2A-D2), play significant roles in modulating neurotransmission and neuroinflammation, which has been explained in detail in Table 1. For example, dopamine-glutamate receptor complexes are crucial for cognitive function and synaptic plasticity, while adenosine-dopamine receptor interactions regulate motor control and are implicated in PD pathology [20].
Various types of heteroreceptors and their role in neuroinflammation
| Heteroreceptor complexes | Structure and mechanism | Neuroinflammatory role | Associated disorders | References |
|---|---|---|---|---|
| A2A-D2 (adenosine-dopamine) | The A2A receptor (GPCR) forms heteromeric complexes with the D2 receptor, leading to an antagonistic interaction where A2A activation inhibits D2 signaling. | Enhances neuroinflammation by inhibiting dopaminergic neurotransmission, leading to microglial activation and neurodegeneration. | Parkinson’s disease, Huntington’s disease | [106] |
| 5-HT2A-D2 (serotonin-dopamine) | Interaction between serotonin 5-HT2A and dopamine D2 receptors modulates dopaminergic neurotransmission and immune signaling. | Dysregulation contributes to aberrant dopamine signaling, leading to heightened neuroinflammatory responses in psychiatric disorders. | Schizophrenia, depression, and bipolar disorder | [107] |
| D2-NMDA (dopamine-glutamate) | Dopamine D2 receptors modulate NMDA receptor function, affecting excitatory neurotransmission and synaptic plasticity. | Dysregulation leads to excessive glutamate excitotoxicity, oxidative stress, and chronic neuroinflammation. | Alzheimer’s disease, schizophrenia, stroke | [30] |
| CB1-D2 (cannabinoid-dopamine) | CB1 cannabinoid receptors interact with D2 dopamine receptors to regulate inflammatory and neuroprotective pathways. | Impairment of CB1-D2 signaling contributes to excessive immune activation, neuroinflammation, and neurodegenerative processes. | Multiple sclerosis, neuroinflammation, anxiety disorders | [108] |
| mGluR5-D2 (metabotropic glutamate-dopamine) | mGluR5 modulates D2 receptor activity, influencing synaptic plasticity and neuroimmune responses. | Aberrant crosstalk leads to excitotoxicity, oxidative stress, and neuroinflammatory damage. | Autism spectrum disorder, schizophrenia, epilepsy | [109] |
| TrkB-NMDA (tropomyosin receptor kinase B-glutamate) | TrkB, activated by BDNF, interacts with NMDA receptors to regulate synaptic strength and neuroprotection. | Dysregulation results in impaired neurotrophic signaling, increased oxidative stress, and inflammatory responses. | Major depressive disorder, Alzheimer’s disease | [110] |
| P2X7-NMDA (Purinoceptor-Glutamate) | P2X7 receptor, an ATP-gated ion channel, modulates NMDA receptor activity and glial cell function. | Overactivation leads to excessive calcium influx, neuronal death, and chronic neuroinflammation. | Multiple sclerosis, Amyotrophic lateral sclerosis (ALS) | [111] |
| α7nAChR-NMDA (nicotinic acetylcholine-glutamate) | Nicotinic α7 receptor modulates NMDA receptor-mediated synaptic transmission and anti-inflammatory responses. | Dysregulation results in impaired cholinergic anti-inflammatory signaling and neurotoxicity. | Alzheimer’s disease, schizophrenia, Parkinson’s disease | [112] |
| CXCR4-CCR5 (chemokine receptors) | CXCR4 and CCR5 heterodimerization regulate neuroimmune signaling and leukocyte infiltration in the CNS. | Hyperactivation promotes neuroinflammatory cascades, contributing to neuronal damage. | HIV-associated neurocognitive disorder, multiple sclerosis | [113] |
| GPR37-D2 (G-protein coupled receptor 37-dopamine) | GPR37 interacts with D2 receptors, modulating dopaminergic transmission and neuroinflammation. | Dysfunction contributes to dopaminergic neurodegeneration and inflammatory signaling. | Parkinson’s disease, schizophrenia | [114] |
GPCRs: G-protein-coupled receptors; NMDA: N-methyl-D-aspartate; BDNF: brain-derived neurotrophic factor; CNs: central nervous system
Heteroreceptor complexes function through various crosstalk mechanisms that influence receptor signaling, trafficking, and neurotransmitter dynamics. One key mechanism is allosteric modulation, where ligand binding to one receptor induces conformational changes in the other, altering its binding affinity and signaling efficiency [33]. For instance, in the A2A-D2 receptor complex, adenosine receptor activation reduces dopamine D2 receptor signaling, which has implications for PD [34]. Another mechanism involves direct protein-protein interactions, where physical contact between receptor domains facilitates cooperative signaling and synaptic stability. This is observed in the dopamine D1-NMDA receptor complex, which enhances NMDA receptor-mediated synaptic plasticity, essential for learning and memory [35].
G-protein coupling interference is another critical mechanism in heteroreceptor interactions, particularly among G-protein-coupled receptors (GPCRs). When receptors form complexes, their G-protein coupling preferences can shift, leading to altered intracellular signaling [36]. For example, in serotonin-dopamine (5-HT2A-D2) complexes, serotonin receptor activation redirects dopamine D2 receptor signaling from the Gi pathway to alternative intracellular cascades, affecting mood regulation and schizophrenia pathology [37]. Additionally, heteroreceptor complexes influence second messenger modulation by altering intracellular pathways such as cyclic adenosine monophosphate (cAMP) and inositol triphosphate (IP3), leading to changes in neurotransmitter release and synaptic plasticity [38]. Another form of crosstalk occurs through receptor trafficking and internalization, where one receptor modulates the internalization and recycling of another. This is evident in the adenosine A2A-dopamine D2 complex, where A2A receptor activation promotes the internalization of the dopamine D2 receptor, leading to altered dopamine signaling and motor control [39].
Heteroreceptor complexes regulate multiple signaling pathways that affect neuronal function, synaptic plasticity, and neuroinflammation. One of the key pathways involved is the cAMP-protein kinase A (PKA) pathway, which plays a central role in neurotransmitter signaling [40]. Heteroreceptor complexes often modulate cAMP levels, thereby influencing PKA activity. In the adenosine A2A-dopamine D2 complex, A2A receptor activation increases cAMP levels, leading to PKA activation, which inhibits dopamine D2 receptor signaling [41]. This mechanism is crucial in PD, where excessive A2A receptor activation contributes to motor dysfunction. Similarly, serotonin-dopamine heteroreceptor interactions can modulate cAMP levels, affecting mood regulation and response to antipsychotic medications [42].
Another key pathway regulated by heteroreceptor complexes is the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway, which is essential for neuronal survival, plasticity, and inflammatory responses. The dopamine D1-NMDA receptor complex enhances NMDA receptor signaling through ERK phosphorylation, promoting synaptic strengthening and memory formation [43]. However, excessive activation of this pathway can also contribute to excitotoxicity in neurodegenerative diseases [44]. The adenosine A2A-dopamine D2 complex, in contrast, can suppress ERK activation, impacting neuronal survival in conditions such as Huntington’s disease and AD [45].
The phosphoinositide 3-kinase (PI3K)-Akt pathway is another crucial signaling mechanism influenced by heteroreceptor complexes. This pathway is involved in cell survival, neuroprotection, and inflammatory responses [46]. Glutamate-metabotropic receptor complexes, for instance, activate PI3K-Akt signaling, protecting neurons from excitotoxicity and reducing neuroinflammatory responses. GABAergic interactions also modulate Akt activity, maintaining excitatory-inhibitory balance and preventing excessive neuronal excitability, which is particularly relevant in epilepsy and neurodevelopmental disorders [47].
Calcium-dependent signaling pathways are also significantly regulated by heteroreceptor complexes. Calcium signaling is critical for synaptic function, neurotransmitter release, and plasticity [48]. Dopamine-glutamate receptor complexes, such as D1-NMDA interactions, potentiate calcium influx, enhancing synaptic plasticity but also increasing the risk of excitotoxic damage in neurodegenerative diseases [49]. Similarly, serotonin-glutamate receptor interactions influence calcium-dependent neurotransmission, which plays a role in mood regulation and neuropsychiatric disorders such as depression and schizophrenia [50].
Heteroreceptor complexes play a crucial role in neuroinflammation, as their dysfunction can lead to excessive cytokine production, oxidative stress, and synaptic abnormalities. In AD, disrupted dopamine-glutamate interactions contribute to cognitive decline and neuroinflammatory responses [51]. In PD, imbalances in adenosine-dopamine heteroreceptor interactions lead to motor dysfunction and neurodegeneration. In schizophrenia, dysregulated serotonin-dopamine receptor interactions play a key role in altered neurotransmission and neuroinflammation, influencing disease progression and therapeutic outcomes [52].
Targeting heteroreceptor complexes through pharmacological interventions is a promising approach to restoring receptor function and mitigating neuroinflammation-driven diseases. By developing drugs that selectively modulate heteroreceptor interactions, researchers aim to fine-tune neurotransmission and reduce the inflammatory burden in neurodegenerative and neuropsychiatric disorders. Understanding the structure, function, and signaling mechanisms of these complexes is essential for advancing therapeutic strategies that address the complexities of brain disorders [53].
AD is a progressive neurodegenerative disorder characterized by amyloid-beta (Aβ) plaque accumulation, tau protein hyperphosphorylation, synaptic dysfunction, and chronic neuroinflammation. The role of heteroreceptor complexes in AD pathology is increasingly recognized, particularly in relation to neurotransmitter dysregulation and inflammatory responses [54]. Dopamine-glutamate heteroreceptor complexes, such as the D1-NMDA and D2-NMDA receptor interactions, are crucial for synaptic plasticity and cognitive function. In AD, these complexes are disrupted due to excessive glutamate excitotoxicity and reduced dopamine signaling, leading to impaired synaptic transmission and neuronal loss. The dysregulation of the D1-NMDA receptor complex exacerbates synaptic weakening, further contributing to cognitive deficits in AD patients [55].
Adenosine-dopamine heteroreceptor complexes, particularly the A2A-D2 complex, also play a significant role in AD-associated neuroinflammation. The overactivation of A2A receptors in response to chronic neuroinflammatory stimuli inhibits D2 receptor signaling, leading to impaired cognitive and motor functions [56]. The involvement of these receptor interactions in neuroinflammation suggests that targeting A2A receptors could be a potential therapeutic approach to modulate neuroinflammatory cascades and improve neurotransmitter balance in AD patients. Additionally, serotonin-dopamine heteroreceptor complexes (5-HT2A-D2) contribute to AD pathology by altering neurotransmitter homeostasis and inflammatory responses. Dysregulated 5-HT2A receptor activity is associated with increased tau aggregation and neuroinflammatory processes, making these heteroreceptor interactions a promising target for AD interventions [57].
PD is a neurodegenerative disorder characterized by progressive loss of dopaminergic neurons in the substantia nigra and chronic neuroinflammation. The dysregulation of heteroreceptor complexes, particularly those involving dopamine and adenosine receptors, is a key contributor to PD pathophysiology. The A2A-D2 heteroreceptor complex is critically involved in the regulation of motor control, as A2A receptor overactivation suppresses dopamine D2 receptor function, leading to motor deficits [58]. In PD, excessive adenosine A2A receptor activity enhances neuroinflammatory signaling through microglial activation and pro-inflammatory cytokine release, exacerbating neuronal loss and motor impairments. Pharmacological blockade of A2A receptors has been explored as a therapeutic approach to restore D2 receptor function and reduce neuroinflammation [59].
Dopamine-glutamate heteroreceptor complexes, such as D1-NMDA and D2-NMDA interactions, are also disrupted in PD, contributing to cognitive impairments and excitotoxic damage. The loss of dopaminergic input in PD alters NMDA receptor activity, leading to excessive calcium influx and oxidative stress, further promoting neurodegeneration. Additionally, serotonin-dopamine heteroreceptor complexes (5-HT2A-D2) play a role in PD-related non-motor symptoms, including depression and cognitive decline [60]. The dysregulation of these receptor interactions contributes to altered neurotransmitter signaling and inflammation, making them potential targets for adjunctive therapies in PD treatment. The modulation of heteroreceptor complexes offers a promising strategy to mitigate neuroinflammation and neurodegeneration in PD patients [61].
MS is a chronic autoimmune-mediated neuroinflammatory disorder characterized by demyelination, axonal damage, and neurodegeneration. The role of heteroreceptor complexes in MS pathology is gaining attention, particularly regarding neurotransmitter dysregulation and immune system interactions. Glutamate-dopamine heteroreceptor complexes, such as D1-NMDA and D2-NMDA interactions, are implicated in MS-related excitotoxicity and neuroinflammation [62]. The excessive activation of NMDA receptors due to dysregulated glutamate signaling contributes to oligodendrocyte damage and demyelination, leading to disease progression [63].
Adenosine-dopamine heteroreceptor complexes (A2A-D2) are also involved in MS-related neuroinflammation. A2A receptor activation promotes microglial activation and pro-inflammatory cytokine release, exacerbating neuroimmune responses. The inhibition of A2A receptors has been proposed as a potential therapeutic approach to modulate neuroinflammatory pathways and protect against demyelination in MS patients [64]. Additionally, serotonin-dopamine heteroreceptor interactions (5-HT2A-D2) are linked to mood disturbances and cognitive impairments observed in MS. The dysregulation of these receptor complexes affects neurotransmitter balance, leading to increased neuroinflammatory responses and exacerbation of psychiatric symptoms. Targeting heteroreceptor complexes to modulate neurotransmitter and immune interactions presents a novel approach for mitigating neuroinflammation and neurodegeneration in MS patients [65].
Neuroinflammation plays a significant role in psychiatric disorders, including depression and schizophrenia, where heteroreceptor complexes are key regulators of neurotransmitter interactions. In depression, serotonin-dopamine heteroreceptor complexes (5-HT2A-D2) are crucial for mood regulation and cognitive function. The dysregulation of 5-HT2A receptor activity in response to chronic stress and neuroinflammation contributes to altered dopamine signaling, leading to depressive symptoms [66]. The interaction between serotonin and dopamine receptors modulates neuroinflammatory responses, and abnormalities in these complexes are linked to increased levels of pro-inflammatory cytokines, oxidative stress, and neuronal dysfunction. Pharmacological agents targeting these receptor interactions, such as serotonin-dopamine modulators, have shown promise in alleviating depressive symptoms by restoring neurotransmitter balance and reducing inflammation [67].
In schizophrenia, dopamine-glutamate heteroreceptor complexes, such as D2-NMDA interactions, play a crucial role in synaptic plasticity and cognitive function. The dysfunction of these complexes contributes to altered glutamate signaling, leading to excitotoxic damage and neuroinflammatory processes [68]. The dysregulation of dopamine-serotonin interactions (5-HT2A-D2) is also implicated in schizophrenia-related cognitive deficits and psychotic symptoms. Increased serotonin receptor activity negatively affects dopamine transmission, contributing to impaired cognitive processing and neuroinflammatory responses. Antipsychotic medications targeting these heteroreceptor interactions aim to restore neurotransmitter homeostasis and reduce neuroinflammation, providing therapeutic benefits in schizophrenia treatment [69].
Heteroreceptor complexes also play a role in bipolar disorder and anxiety-related conditions, where neurotransmitter imbalance and neuroinflammatory mechanisms contribute to disease pathology. The modulation of glutamate-dopamine and serotonin-dopamine receptor interactions influences mood regulation, synaptic plasticity, and inflammatory pathways. Emerging research suggests that targeting heteroreceptor complexes could provide novel therapeutic strategies for psychiatric disorders by reducing neuroinflammation and restoring neurotransmitter function [70]. Understanding the role of these receptor interactions in psychiatric conditions is essential for developing precision medicine approaches that address the underlying neurobiological mechanisms of mental health disorders [71].
Neuroinflammation is a complex physiological process involving interactions between neurons, glial cells, and immune signaling pathways. Heteroreceptor complexes play a crucial role in modulating neuroimmune responses by regulating synaptic transmission, neurotransmitter release, and inflammatory signaling cascades [72]. These receptor complexes, consisting of two or more different neurotransmitter receptors physically interacting, influence intracellular signaling pathways that mediate neuroinflammation. Disruptions in heteroreceptor crosstalk can lead to aberrant immune activation, contributing to chronic neuroinflammatory states that underlie various neurological disorders [73].
In conditions such as AD, PD, and MS, heteroreceptor complexes act as regulators of neuroimmune interactions. Dysregulated heteroreceptor complexes, particularly those involving dopamine (D2), adenosine (A2A), serotonin (5-HT2A), and glutamate (NMDA) receptors, contribute to altered immune responses [74]. These interactions affect the release of pro-inflammatory cytokines, reactive oxygen species, and neurotoxic mediators, leading to neuronal dysfunction and neurodegeneration. Thus, understanding the role of heteroreceptor complexes in neuroimmune signaling provides valuable insights into novel therapeutic approaches targeting neuroinflammatory pathways [75].
Microglia and astrocytes are the primary immune cells of the CNS, playing an integral role in maintaining homeostasis and responding to neuroinflammatory stimuli. Heteroreceptor complexes modulate the activation state of these glial cells, influencing their capacity to regulate synaptic function and neuroinflammatory responses [76]. In pathological conditions, dysregulated receptor crosstalk between neurons and glial cells exacerbates inflammation, leading to progressive neuronal damage [77].
Microglial activation is influenced by heteroreceptor interactions involving purinergic (P2X7), dopamine (D2), and adenosine (A2A) receptors. Under normal physiological conditions, these complexes maintain microglial homeostasis by balancing pro-inflammatory and anti-inflammatory responses [78]. However, in neurodegenerative diseases, excessive activation of A2A receptors, for instance, leads to heightened microglial reactivity, promoting the release of TNF-α and IL-1β, which contribute to neurotoxicity. Similarly, dopamine-glutamate heteroreceptor complexes influence astrocytic function, modulating glutamate uptake and preventing excitotoxicity. When disrupted, these interactions impair astrocytic glutamate clearance, exacerbating neuroinflammatory damage [79].
Astrocytes, in particular, contribute to neuroinflammation by releasing pro-inflammatory cytokines and modulating blood-brain barrier integrity. The crosstalk between serotonin (5-HT2A) and glutamate receptors in astrocytes regulates inflammatory responses, influencing cytokine production and gliosis [80]. The involvement of heteroreceptor complexes in glial function highlights their significance in shaping neuroimmune responses and presents potential targets for mitigating neuroinflammation-associated neurodegeneration [81].
Heteroreceptor complexes influence the expression and release of cytokines and other inflammatory markers, serving as mediators of neuroinflammation [82]. These receptor complexes modulate intracellular signaling pathways that regulate the balance between pro-inflammatory and anti-inflammatory cytokine production. Dysregulation of these interactions leads to excessive inflammation, a hallmark of neurodegenerative and psychiatric disorders [83].
The activation of heteroreceptor complexes, such as A2A-D2 and 5-HT2A-D2, alters immune responses by modulating the activity of NF-κB, MAPKs, and Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathways [84]. These intracellular signaling cascades govern the production of cytokines such as IL-6, TNF-α, and IL-1β. Excessive cytokine release in response to dysregulated receptor crosstalk contributes to chronic neuroinflammation, exacerbating neuronal damage and synaptic dysfunction [85].
Furthermore, heteroreceptor complexes interact with chemokines and other inflammatory mediators that regulate immune cell infiltration into the CNS. In MS, for example, heteroreceptor interactions influence the migration of peripheral immune cells across the blood-brain barrier, contributing to neuroinflammatory demyelination [86]. Similarly, in psychiatric disorders such as depression and schizophrenia, heteroreceptor dysregulation leads to altered cytokine profiles, influencing neurotransmitter function and exacerbating disease pathology [87].
Heteroreceptor complexes represent a promising target for drug development, particularly in the treatment of neuroinflammatory and neurodegenerative disorders. These complexes, formed by interactions between different neurotransmitter receptors, play a crucial role in modulating synaptic transmission, neuroimmune responses, and inflammatory signaling pathways [88]. Given their influence on neuronal homeostasis and immune activation, pharmacological interventions aimed at restoring the balance of heteroreceptor signaling may provide novel therapeutic avenues for conditions such as AD, PD, MS, and psychiatric disorders [89].
One of the key advantages of targeting heteroreceptor complexes is the ability to fine-tune neurotransmitter systems with greater specificity than traditional single-receptor-targeting drugs. For instance, the dopamine-glutamate heteroreceptor complexes, including D2-NMDA interactions, are involved in cognitive and motor functions. Dysregulation of these complexes contributes to excitotoxicity and neurodegeneration, making them potential targets for drugs that modulate both dopamine and glutamate signaling simultaneously [90]. Similarly, the adenosine-dopamine heteroreceptor complex (A2A-D2) has been implicated in PD, where excessive adenosine A2A receptor activity inhibits dopamine D2 receptor function, exacerbating motor impairments and neuroinflammation. Drugs that selectively modulate A2A receptor activity, such as istradefylline, have demonstrated therapeutic potential in alleviating symptoms of PD by restoring dopaminergic signaling and reducing neuroinflammation [91].
Another promising approach is the modulation of serotonin-dopamine heteroreceptor complexes (5-HT2A-D2), which play a role in psychiatric disorders such as schizophrenia and depression. Atypical antipsychotics, such as clozapine and risperidone, target these receptor interactions, leading to improved treatment efficacy compared to traditional dopamine-based antipsychotics. By refining drug design to selectively target heteroreceptor complexes, researchers can develop more effective and less side-effect-prone treatments for neuropsychiatric and neurodegenerative conditions [92].
The emergence of allosteric modulators and biased agonists has revolutionized the field of receptor pharmacology, offering new strategies to manipulate heteroreceptor complex function. Unlike traditional orthosteric ligands, which directly activate or inhibit receptors, allosteric modulators bind to distinct sites on the receptor complex to enhance or diminish receptor responses in a highly selective manner. This specificity reduces off-target effects and allows for more precise control over receptor signaling pathways [93].
Positive allosteric modulators (PAMs) and negative allosteric modulators (NAMs) have shown potential in fine-tuning heteroreceptor activity in neuroinflammatory disorders. For example, PAMs targeting metabotropic glutamate receptors (mGluRs) have been explored as potential treatments for neurodegenerative diseases by modulating glutamate-dopamine receptor interactions and reducing excitotoxicity. Similarly, A2A receptor antagonists, acting as NAMs, have demonstrated efficacy in preclinical and clinical models of PD by preventing excessive adenosine-mediated inhibition of D2 receptor activity [94].
Biased agonism, or ligand-directed signaling, is another promising approach for targeting heteroreceptor complexes. This concept involves designing drugs that preferentially activate specific signaling pathways while avoiding pathways associated with adverse effects. For example, dopamine D2 receptor-biased agonists have been developed to selectively engage beneficial G-protein signaling while minimizing β-arrestin-mediated side effects, which are linked to dyskinesia and cognitive dysfunction [95]. Such approaches could enhance the therapeutic index of drugs targeting heteroreceptor complexes in neuroinflammatory and neuropsychiatric disorders [96].
In addition to small-molecule modulators, peptide-based therapeutics and monoclonal antibodies are being investigated as potential modulators of heteroreceptor function. These biologics offer high specificity and can be engineered to disrupt or stabilize receptor-receptor interactions, providing novel therapeutic strategies for neuroinflammatory diseases [97].
The clinical translation of heteroreceptor complex-targeting drugs is still in its early stages, but several promising candidates are being explored in clinical trials. Adenosine A2A receptor antagonists, such as istradefylline, have already been approved for the treatment of PD, providing proof of concept that targeting heteroreceptor interactions can yield therapeutic benefits [98]. Additionally, serotonin-dopamine receptor modulators, including pimavanserin, have been developed for the treatment of PD psychosis and are being investigated for other neuropsychiatric disorders [99].
Several ongoing clinical trials are evaluating allosteric modulators targeting heteroreceptor interactions in neuroinflammatory conditions. For example, mGluR5 allosteric modulators are being tested for their ability to modulate glutamate-dopamine receptor interactions in schizophrenia and depression. Similarly, biased agonists targeting dopamine and serotonin receptors are being explored for their potential to improve therapeutic outcomes in psychiatric and neurodegenerative diseases while reducing side effects [100].
Future research in this area will focus on refining our understanding of heteroreceptor complex dynamics and identifying novel targets for therapeutic intervention. Advances in Boolean analysis, structural biology, cryo-electron microscopy, and computational modeling are providing new insights into receptor-receptor interactions, enabling the rational design of drugs with enhanced specificity and efficacy [101, 102]. Additionally, the development of gene editing technologies and RNA-based therapies holds promise for modulating heteroreceptor function at the genetic and epigenetic levels [103].
Personalized medicine approaches will also play a key role in the future of heteroreceptor-targeted therapies. By utilizing biomarkers and neuroimaging techniques, clinicians can identify patient-specific alterations in heteroreceptor signaling and tailor treatments accordingly [104]. This precision medicine approach has the potential to improve treatment outcomes for patients with neuroinflammatory and neurodegenerative disorders [105].
Despite significant advancements, numerous open research questions remain regarding the precise molecular mechanisms underlying heteroreceptor complex formation, function, and dysregulation in neuroinflammatory disorders. The dynamic nature of these complexes, their tissue-specific expression, and the influence of genetic and environmental factors require further elucidation. Future studies must explore how heteroreceptor interactions contribute to disease pathophysiology and whether they can serve as reliable biomarkers for early diagnosis and disease progression. Additionally, translational applications of heteroreceptor-targeted therapies must be optimized through rigorous preclinical validation and well-designed clinical trials. The development of novel pharmacological tools, including high-affinity allosteric modulators and biased agonists, holds promise for precise therapeutic modulation with minimal adverse effects. As the field progresses, integrating cutting-edge technologies such as cryo-electron microscopy, optogenetics, and single-cell transcriptomics will provide deeper insights into heteroreceptor signaling networks, paving the way for innovative drug discovery approaches. This emerging understanding has the potential to revolutionize neuropharmacology by enabling highly targeted, mechanism-based therapies tailored to individual patient profiles. The convergence of basic and clinical research will be instrumental in translating these findings into effective treatments, ultimately improving patient outcomes in neuroinflammatory and neurodegenerative diseases. By bridging molecular neuroscience with clinical innovation, the study of heteroreceptor complexes stands at the forefront of transformative advancements in personalized medicine and neurological care.
AD: Alzheimer’s disease
AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
cAMP: cyclic adenosine monophosphate
CNS: central nervous system
ERK: extracellular signal-regulated kinase
GABA: gamma-aminobutyric acid
GPCR: G-protein-coupled receptor
IL: interleukin
MAPK: mitogen-activated protein kinase
MS: multiple sclerosis
NAMs: negative allosteric modulators
NF-κB: nuclear factor kappa B
NMDA: N-methyl-D-aspartate
PAMs: positive allosteric modulators
PD: Parkinson’s disease
PI3K: phosphoinositide 3-kinase
PKA: protein kinase A
TNF-α: tumor necrosis factor-alpha
We, the authors, sincerely acknowledge REVA University for providing an opportunity and platform for research.
NSK: Conceptualization, Investigation, Writing—original draft. SR: Validation, Writing—review & editing, Supervision. Both authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3083
Download: 27
Times Cited: 0
Ahmed Hasbi, Susan R. George
Diego Guidolin ... Luigi F. Agnati
Adèle Vilette ... Peter Vanhoutte
