Affiliation:
1Medical University of Innsbruck, A-6020 Innsbruck, Austria
2Current address: Rosengasse 5, A-6060 Hall, Austria
Email: schirmer.michael@icloud.com
ORCID: https://orcid.org/0000-0001-9208-7809
Affiliation:
3Department of Orthopaedics and Traumatology, Medical University of Innsbruck, A-6020 Innsbruck, Austria
Email: johannes.pallua@i-med.ac.at
ORCID: https://orcid.org/0000-0003-0203-213X
Explor Musculoskeletal Dis. 2024;2:82–91 DOI: https://doi.org/10.37349/emd.2024.00037
Received: October 01, 2023 Accepted: November 21, 2023 Published: April 08, 2024
Academic Editor: Rebecca Grainger, University of Otago, New Zealand
The article belongs to the special issue Digital health technologies in rheumatology: emerging evidence and innovation
Recent developments in digital health technologies are overwhelming, and their use in routine work is still difficult to anticipate. This narrative review summarizes the concept of consecutive cohorts in the literature, together with local research experiences in consecutive rheumatic outpatients. Digital health techniques have to reflect the clinicians’ needs, support real-life care of patients, and allow for the specific assessment of quality parameters fulfilling the Donabedian aspect of qualified health care, using quality indicators to improve health care and research. Rapidly growing observational cohorts will perform best to provide follow-up data as the basis for further development of healthcare approaches for rheumatic patients. The challenges of a selection bias, patients with limited disease expression, and chances of early detection of patients with rare diseases are addressed. For research purposes, sequential analyses with growing cohort size, comparative cross-sectional studies with sequential hypothesis testing and other prognostic, diagnostic, and therapeutic aspects of patient management can be performed. With the support of new technologies, young clinicians can easily approach such clinical topics, and learn about clinical data analyses. The use of quality standards as proposed in international recommendations for diagnostic issues and classification criteria, management recommendations, monitoring, and training issues can be supported by digital technologies. In conclusion, collaborative projects allow detailed clinical analyses of large cohorts, but local initiatives can prepare these co-operations, provide first local logistics and research experiences, and teach clinicians how to perform clinical research. Digital health technologies will strongly support these local initiatives.
Assessment of quality in rheumatology is challenging for several reasons. First, more than 200 different diagnoses present with clinical signs and symptoms with varying severity and presentation time, based on other, still not always fully understood pathogenetic backgrounds, and with single or mostly multiorgan involvement. Second, awareness and recognition of more than 5,000 additional rare genetic diseases with musculoskeletal system involvement are necessary and can be considered the second important aspect of clinical quality for practicing rheumatologists. Therefore, multiple aspects have to be considered when trying to assess and potentially improve the quality of care in rheumatology, including the use of quality indicators for clinical routine, implementation of treat-to-target (T2T) as a treatment strategy, a predefined evidence-based role of nursing in rheumatologic care, the accomplishment of additional quality aspects like polypharmacy, and data acquisition to provide sufficient quality indicators [1].
To tackle this huge challenge of quality of care, digital health solutions are considered potential support systems for the clinical, laboratory, and imaging evaluation of individual patients. These solutions can help improve evidence-based clinical decision-making and aid in documenting patients’ follow-ups with physicians. Additionally, they can provide important information from medical product information or orphan disease registries, and international scientific communities like the European Alliance of Associations for Rheumatology (EULAR), for physicians and patients to consider. Digital health can be defined as “the use of information and communications technologies in medicine and other health professions to manage illnesses and health risks and to promote wellness”, including “the use of wearable devices, mobile health, telehealth, health information technology, and telemedicine” [2]. Indeed, it has been shown that digital technologies offer several opportunities for rheumatology, but several challenges remain to be solved until successful use of these opportunities in routine care [3]. New dimensions of quality care will become possible after implementing such digital health technologies [4]. It can be anticipated that (1) the use of artificial intelligence will support diagnostic, prognostic and management decisions [5]; (2) multidisciplinary, long-term co-operations of digital health technicians with clinical health care workers, quality managers, researchers including epidemiologists, teaching experts and lawyers will develop to contribute to digital health systems; and (3) such multifunctional digital health systems will result in increased effectiveness of rheumatologists provision of health care, together with improved evidence-based delivery of quality care [6, 7].
The challenges of implementing digital health technologies into clinical care are multifold, including technical and essential clinical issues [3]. With ongoing changes in approved medications with indications and contraindications, evidence-based national and international recommendations, recommended quality parameters, and the involvement of patients and varying healthcare providers, the technical challenges often outweigh the financial resources. Besides, from the clinical perspective, with more than 200 different rheumatic diagnoses, specificity is needed for each patient to ensure rapid recognition of clinical situations and adequate response to pathological changes. Data from unselected, consecutive cohorts provides many opportunities to assess real-life patients’ care by practicing physicians and allows the development of artificial intelligence for research on diagnosis, prognosis, and management of specific diseases and situations.
This narrative review aims to summarize the concept of consecutive cohorts with local research experiences in consecutive rheumatic outpatients, together with a proposal to develop digital health technologies in a clinical setting.
The main advantage of digital health technologies should be the facilitation of fast and specific documentation of routine clinical work, thus empowering clinicians and cooperating healthcare workers to improve the quality of care for individual patients. In recent years, Donabedian’s approach to quality assessment has become widely accepted for defining and evaluating quality in medical care [8]. According to Donabedian’s conceptual model, key performance indicators are classified differently by referring to the structure, which describes the context in which health care is delivered, the processes, including all transactions between patients and providers throughout the healthcare continuum and outcomes referring to the impact of health care on the health status of patients [9]. Donabedian’s approach aims at measuring quality across all three different dimensions of quality: structure, process, and outcome. While previous studies on quality improvement have primarily focused on process and outcome quality, the dimension of structural quality has been given less attention [10, 11]. Besides, there is a growing need for a paradigm shift in rheumatology, with the future focus of healthcare not just defined by the physical aspect but also including the psychological and social issues of the patients, in line with the comprehensive definition of health by the World Health Organization (WHO) [12]. This calls for developing and promoting new approaches by healthcare providers, policymakers, and health insurers.
Digital health technologies will provide the opportunity to specify the measurement and documentation of all these different quality aspects, bridging gaps between clinical work and quality assessment, between patients and health care providers, and between various medical disciplines. A conceptual framework becomes necessary to define the relevant facts for data collection.
Many healthcare organizations have developed quality indicators to monitor, measure, and manage the performance of their health systems [13, 14]. This ensures effectiveness, efficiency, equity, and quality, as healthcare organisations are expected to achieve outcomes consistent with their established goals and quality standards (QS). From the medical perspective, all patients should reach the goals of health as defined by the WHO, thus not excluding patients with pathologic signs and symptoms but without fulfilling any diagnostic criteria. Therefore, it is crucial to develop strategic performance indicators that reflect the global performance of healthcare organizations [14] and meet the requirements of clinical relevance, scientific acceptability, and applicability [15]. They must be scientifically reliable and validated by experts [16] after a well-described methodological development process [17]. Hospitals may then use these parameters to monitor and evaluate performance against benchmark values or standards, showing trends and indicating possible changes over time. They also help compare results to standards or similar organizations and improve the services provided.
In rheumatology, performance indicators are used to measure the success of patient therapy. This is done using the “T2T” concept, which has been defined primarily for patients with rheumatoid arthritis (RA), spondyloarthritis (SpA), and arthritis urica to reach remission or at least low disease activity [18]. If well-documented long-term follow-ups of patients also fulfill the Donabedian aspect of qualified health care, these data will provide more perspectives for clinical work, research purposes, and quality reporting. Overall, digital health techniques have to reflect all the clinicians’ needs, support real-life care of the patients, and allow specific assessment of quality parameters fulfilling the Donabedian aspect of qualified health care. Taken together, this approach will stimulate new clinical research approaches.
It can be anticipated that observational cohorts with consecutive patients will perform best to provide such follow-up data as the basis for further development of healthcare approaches for rheumatic patients. Well-prepared data can then be used for different clinical, quality, research, and educational purposes, as shown in Figure 1.
Opportunities for data from patients with rheumatic diseases to be used to improve rheumatological health care. Digital health technologies include optimized data acquisition and preparation for all these purposes
Documentation of routine clinical work in consecutive patients leads to an observational cohort without further preselection of the recruited patients. It is important to note that without consecutive screening, patients recruited in trials may not be representative of the disease under investigation, with younger age, male gender, and lower risk groups being more prevalent in conventional cardiovascular trials for example [19]. These authors concluded that consecutive screening should be mandatory in all future clinical trials. Trials without such consecutive screening are considered less reliable and valuable [20]. For this purpose, the research group of our rheumatic outpatient clinic planned a prospective observational trial to recruit all consecutive patients after informed and written consent. The trial was approved by the ethical committee of the Medical University [AN 2017-0041 37074.18 453/AM2 (4581a)].
However, selection biases may occur and must be identified and reported, as they influence the outcome of the statistical analyses and the drawn conclusions. In observational trial of a rheumatic outpatient clinic, a possible selection bias may be the co-existing outpatient clinic for dermatology, ophthalmology, and gastroenterology. Other manifestations of systemic disease may lead a patient to successful treatment of the other comorbidity with subsequent improvement of the rheumatic problem, too. For example, this may apply for patients diagnosed with SpA related to psoriatic disease, uveitis, or inflammatory bowel disease colitis, who were successfully treated using biological or targeted synthetic disease-modifying antirheumatic drugs and, therefore, did not show up in the rheumatic out-patient clinic.
Also, some patients may not provide informed and written consent. This percentage should account for an estimated less than 5% of all patients to provide a reliable base for clinical research.
For interventional trials, it is recommended to randomize patients into treatment and control groups, ensuring the comparability of the two groups regarding their characteristics. For identifying patients with certain diagnostic features, the higher the probability, the larger the size of the consecutive cohort. The clinical, laboratory, and imaging data of these sub-cohorts with specific diagnoses can then be compared. In a consecutive cohort, most patients will show the full spectrum of a disease, but some patients will offer only limited disease expression or even be undiagnosed, fulfilling diagnostic criteria only during follow-up of the disease. Otherwise, some patients with rare manifestations may not show up in such a sub-cohort, but single patients with a less frequent or even orphan or rare disease will show up.
Given the circumstances and localization of the clinical events, such an observational cohort of consecutive patients will rapidly grow. For example, in an ongoing rheumatic out-patient trial, a total of 2,811 patients were recruited and subsequently followed by a single investigator on 1,553 working days between September 2017 and September 2023, with a median of 3 visits [interquartile range (IQR) 1–7] per patient until January 9, 2023 [21]. Inclusion criteria were age over 17 years and the capability to understand the purpose of the study and provide informed consent to the study participation. An additional nine patients drew back their consent after written consent, with data used for the trial only until their withdrawal date. Patients’ data from these and prior visits at this institution were available for the research studies cited in this work and led to a data set of about 20,000 physicians’ reports used for this trial. In this cohort, the primary diagnoses are RA, psoriatic arthritis, and axial SpA accounting for 14.4%, 13.4%, and 11.2% of all diagnoses [21]. These sub-cohorts can then be compared with each other easily.
For this trial, data acquisition was manually performed by students for their diploma works, with all the limitations of possibly missing data and mistakes. Therefore, follow-up studies with larger cohort sizes are difficult to achieve and time-consuming. Based on these and other experiences, such studies are not favoured by clinical researchers, and in the future, with powerful digital health technologies, many analyses can be anticipated with larger cohorts as comparisons during follow-up become easier. Thus, new research and quality assessment dimensions can be developed, even with regular repetitions of low costs.
As a first step of recognizing the value of such a consecutive cohort, single patients with a rare condition, disease, or disease course may be considered for possible publication as a case report. Indeed, from this rheumatic out-patient cohort, cases with significant reduction of pain medication after diagnosis of SpA [22] and the need for accessibility in common variable immunodeficiency with granulomatous-lymphocytic interstitial lung disease (GLILD), who was rapidly and successfully treated with monoclonal antibodies against coronavirus disease 2019 (COVID-19), were already reported [23]. Such case reports may be underrecognized in the literature, but case reports may stimulate the authors to recognize important aspects of their work and the readers to draw attention to specific conditions, diseases, or disease courses. Follow-ups of individual cases may be of specific interest to specialists, then summarized of similar cases to case series even years after publication. Supporting such development by rapid identification and better comparison of health data can be an additional challenge for digital health technologies in the future. Case series with similar disease courses or treatments of patients will further increase the medical community’s knowledge.
For research purposes, cohort studies recruit consecutive patients allowing for cross-sectional observations with or without sequential analysis or sequential hypothesis testing, as the sample size is not fixed in advance. Such research may address prognostic, diagnostic, and therapeutic aspects of patient management. Sequential analyses then provide the opportunity to assess changes during follow-up. From the statistical perspective, with repeated analyses, it is essential to note that as more observations are added, the probability of a Type 1 error increases. Therefore, each interim analysis must adjust the alpha level to control the Type 1 error [24].
The research question to be followed for analyses of cohort data strongly depends on the cohort size. Initially, the focus will be on frequent diseases, and comparisons between patients’ groups from the same cohort but with different diagnoses are avoided. Next, subgroups of inflammatory and non-inflammatory conditions can be formed. Finally, diagnostic sub-groups with the most frequent diseases can be defined.
With larger sub-cohorts, data can be used to compare with national and international data. Such comparisons allow further conclusions and provide critical information for future studies. For example, a specific focus on cardiovascular risk factors in inflammatory joint disease can be performed, comparing data with Spanish and Norwegian data [25], clinical manifestations of Behcet’s disease can be compared with German and Turkish data [26], and comorbidities in RA patients can be compared with data from the international COMORA trial [27, 28]. Thus, data from national and international cohorts allowed both the confirmation of exciting findings and the discussion of divergent results.
The major limitation of such a study is its retrospective approach, with manual search in unstructured data. Such data acquisition is complex, possibly missing data from additional visits to other institutions. Also, the data set can be too small for age and sex adjustments without the option of repeated analyses in a larger cohort during the follow-up of the ongoing observational trial. In case of too small sample sizes, sequential analyses could even allow a conclusion with improved power with a larger cohort size later [24]. Lower financial and/or human costs will apply when using digital health techniques for these purposes.
The strength of cohort studies is undoubtedly the focus on clinical topics by young researchers who otherwise lack research experience. For example, though differences did not reach a significant level the prevalence of cardiovascular disease was calculated as quite high with 8.7% in SpA, 12.8% in psoriatic arthritis, and 18.7% in RA patients which has to be considered when visiting patients [25]. Behcet’s patients of Turkish origin from the non-endemic Innsbruck sub-cohort present differently compared to those living in Turkey [26]. Polypharmacy defined by ≥ 5 drugs was observed by 33.7% of patients with RA in the Middle-European cohort, compared to 61.6% in the literature [29]; and 88.8% of all Middle-European RA patients were in remission or had low disease activity, with hypertension (38.8%) and osteoporosis (30.0%) as the most frequent comorbidities [27]. The potential need for genetic testing is immense, with 57.3% of patients having the potential to benefit from genetic testing according to their diagnosis and treatment and 53.3% of patients with actually performed genetic testing for diagnostic, prognostic, or pharmacogenetic purposes [21]. The list of such topics can be easily extended, and larger cohort sizes will undoubtedly allow more valid conclusions. With the support of new technologies, young clinicians will learn to approach a clinical topic in their cohort much more easily and compare the results with national and international data.
The value of quality assessment is undoubtedly higher when considering disease-specific aspects of a diagnosis. A years-long diagnostic delay from the 1st symptom of inflammatory back pain until diagnosis of SpA may be acceptable compared to a mean international delay of 6.7 years [30], but is unacceptable for patients with RA. In RA, patients have been shown to develop radiographic damage within three months of delayed diagnosis [31].
Only a few disease-specific, international recommendations for improving the quality of health and care services are available. For axial SpA, the Assessment of SpondyloArthritis International Society (ASAS) provided such QS [32]. These recommendations focus on the main aspects of quality improvement: referral times to a rheumatologist, time until a first visit by a rheumatologist, time until diagnosis, monitoring and treatment characteristics, and patient education. Unfortunately, some of the recommended QS are unavailable in the routine clinical assessment, like the time until the general practitioner’s referral to a rheumatologist, the time from referral to the first rheumatologic visit, or the use of the Ankylosing Spondylitis Disease Activity Score (ASDAS) score for monitoring disease activity. Instead, in some institutions, the Bath Ankylosing Spondylitis Disease Activity Score (BASDAI) is still used, which allows rapid decision-making without waiting for laboratory data. Also, physicians’ information about the option of additional medications may not be documented in the charts of all SpA patients with disease activity despite the use of non-steroidal antirheumatic drugs. Accordingly, documentation habits should be adapted to the needs of international recommendations for quality assessments or at least discussed in the international community. Finally published data on all these aspects assessed in rheumatic centers are rare to allow a direct comparison.
Of note, the ASAS report considers the improvement rather than the pure assessment of the quality of health and care services as the primary goal of developing QS. This fact is relevant, especially concerning developing QS over time. Indeed, during the last two decades it appeared in observational cohort, the time from 1st visit to diagnosis in patients with SpA improved, as did the diagnostic delay in patients with RA (Figure 2).
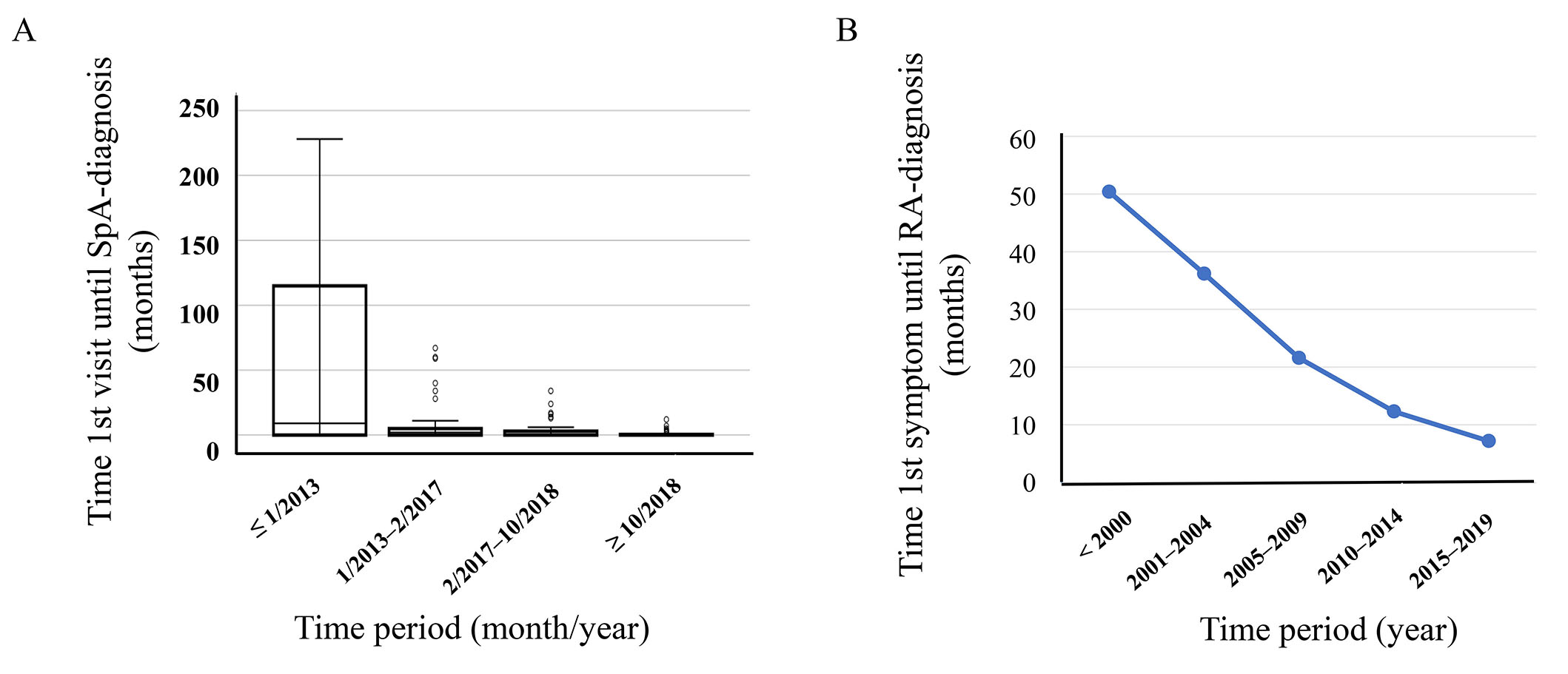
Possible changes of QS during the past two decades. (A) An observational cohort of consecutive patients with improving times from the first visit until the diagnosis of SpA; (B) an observational cohort of consecutive patients with improving times from the first visit until the diagnosis of RA [25]
Over the last few years, a growing list of critical international recommendations has been developed, which will further improve qualified clinical practice. Examples of such recommendations are listed in Table 1.
List of international recommendations relevant for qualified health care to be supported by digital health technologies (for example, as provided for specific conditions by international societies)
| Topics of international recommendations |
|---|
Diagnostic issues and classification criteria: -Awareness campaigns, referral strategies. -Reducing delay in diagnosis progression of psoriasis to psoriatic disease. -Performance of laboratory assays, use of imaging in clinical practice. |
Management recommendations: -Pharmacological management, drug monitoring. -Intraarticular, radionuclide, and surgical therapies. -Non-pharmacological management. |
Monitoring: -Criteria for treatment response, reporting disease activity. -Cardiovascular risk management with blood pressure and lipid management. -Prevention and management of fragility fractures in adults over 50 years. -Vaccination guidelines. -Medication-related side effects (e.g., adverse events by checkpoint inhibitors). |
Specific management issues: -Role of other healthcare workers. -Implementation of self-management strategies. -Remote care, support to participate in paid work. -Patient education, including lifestyle behaviors and work participation. |
Training issues: -Competences in rheumatology specialty training. -Generic core competencies of health professionals. -Mobile health applications for self-management by patients. |
Implementation of all these different recommendations into clinical practice will undoubtedly be a challenge for clinicians and all healthcare workers involved. In parallel, patients will have to be educated and trained on how to use new digital technologies so that they know about their disease, comorbidities, and possible side effects of their medications on a regular, updated basis.
In conclusion, collaborative national or international projects allow for detailed clinical analyses of large cohorts, but local initiatives can prepare these co-operations, provide first local logistics and research experiences, and teach clinicians how to perform clinical research. For successful collaborative projects, unified documentation habits are a condition sine qua non to improve data quality and allow better comparability. Digital health technologies will strongly support these local initiatives.
QS: quality standards
RA: rheumatoid arthritis
SpA: spondyloarthritis
After informed and written consent, the authors thank all patients who participated in this prospective study. Also, the authors thank all students for their diploma works with manual extraction of patients’ data from the hospital information system.
MS: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. LK: Writing—review & editing. JDP: Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The prospective trial to recruit consecutive patients was approved by the local Ethics Committee of the Medical University of Innsbruck [AN 2017-0041 37074.18 453/AM2 (4581a)]. The research complies with the Declaration of Helsinki.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors (Michael Schirmer, schirmer.michael@icloud.com), without undue reservation, to any qualified researcher.
The work was partly funded by Verein zur Förderung der Hämatologie, Onkologie und Immunologie, Innsbruck, Austria. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Hannah Labinsky ... Johannes Knitza
Prakashini Mruthyunjaya ... Debashish Danda
Diego Benavent ... Antonio Gómez-Centeno
Sumaira Farman ... Saira Elaine Anwer Khan
Carlos A. Guillén-Astete ... Mónica Vázquez-Díaz
Judy L. Seraphine, Alvin F. Wells
