Affiliation:
1Department of Physiology, National Institute of Medical Sciences and Research, NIMS University, Jaipur 303121, India
ORCID: https://orcid.org/0000-0002-7656-7033
Affiliation:
1Department of Physiology, National Institute of Medical Sciences and Research, NIMS University, Jaipur 303121, India
Email: ailanivinita@gmail.com
ORCID: https://orcid.org/0000-0001-9317-8059
Affiliation:
2Department of Physiology, Rajarshi Dashrath Autonomous State Medical College, Ayodhya 224133, India
Affiliation:
3Department of Pathology, Rajarshi Dashrath Autonomous State Medical College, Ayodhya 224133, India
ORCID: https://orcid.org/0000-0002-1818-1407
Affiliation:
4Department of Medicine, Rajarshi Dashrath Autonomous State Medical College, Ayodhya 224133, India
Affiliation:
5Department of Plastic Surgery, King George’s Medical University, Lucknow 226003, India
ORCID: https://orcid.org/0000-0001-6032-5630
Affiliation:
6Department of Physiology, Madhav Prasad Tripathi Medical College, Siddharth Nagar 272207, India
Explor Med. 2022;3:368–374 DOI: https://doi.org/10.37349/emed.2022.00099
Received: April 25, 2022 Accepted: May 24, 2022 Published: August 29, 2022
Academic Editor: Laura Sciacca, University of Catania, Italy
Aim: Lactate dehydrogenase (LDH) is an enzyme that acts as a catalyst in the conversion of lactate to pyruvate which is abundantly found in liver, kidney, heart and muscles. Previous studies have all shown a strong positive correlation between muscle fatigue and increased serum LDH levels with type 2 diabetes mellitus but no study has actually assessed the same for prediabetes. The basic objective of this study, thus, is to find out the correlation between muscle fatigue and serum LDH levels in prediabetic individuals.
Methods: A cross-sectional study was conducted in adults between 24–60 years old who were classified as prediabetic individuals as per norms established by American Diabetes Association. A total of 100 prediabetic individuals were selected for the study. Fatigability was calculated as a function of work done by the pleximeter finger of the dominant hand using Mosso’s ergograph. The study was conducted at Rajarshi Dashrath Autonomous State Medical College, Ayodhya.
Results: Out of 100 prediabetic participants, 50% were males with a mean age of 44.14 ± 10.91 years and remaining 50% were females with a mean age of 41.12 ± 11.5 years. Overall, the average work done by the participants was 2.9 ± 1.2 weight lifted•total distance moved (kg•m) with an average serum LDH level of 323.84 ± 26.82 unit/litre (U/L).
Conclusions: This study aimed at assessing the correlation between muscle fatigue and serum LDH levels in prediabetic individuals so that further work can be initiated to improve the quality of life in prediabetics that maybe drastically hampered due to easy fatigability in prediabetic individuals.
Prediabetes has been established as a state of disordered glucose metabolism rather than a distinctive clinical entity that signifies an important risk factor for the development of diabetes [1, 2]. Prediabetes is a serious health condition where blood sugar levels are higher than normal, but not high enough to be diagnosed as the type 2 diabetes. The exact cause of prediabetes is unknown. But family history and genetics appear to play important roles. Five to ten percent of those with prediabetes progress to diabetes every year. Almost 70% of individuals with prediabetes will eventually develop diabetes according to the American Diabetes Association (ADA) expert panel [3]. Prediabetes has been linked with long-term damage, including to your heart, blood vessels and kidneys, even if one hasn’t progressed to type 2 diabetes. Prediabetes is also linked to unrecognized (silent) heart attacks. Lactate dehydrogenase (LDH or LD) is an enzyme found in nearly all living cells. LDH catalyzes the conversion of lactate to pyruvate and back, as it converts nicotinamide adenine dinucleotide (NAD) to NAD+ + hydrogen and vice versa. A dehydrogenase is an enzyme that transfers a hydride from one molecule to another. The main function of LDH is to act as a catalyst in the conversion of lactate into pyruvate and is found in almost all the tissues of the body [4, 5]. LDH has a total of five isoenzymes out of which LDH1 and LDH2 are mainly found in blood while LDH4 and LDH5 mainly exist in saliva [6]. Tesch et al. [7] suggested that LDH activity is correlated to muscle fatigue. Similarly, the aim of this study was to find out the correlation between muscle fatigue and serum LDH levels in prediabetic individuals. This will help to conclude that LDH deficiency or any kind of pH changes might be considered as an adverse outcome of prediabetes in causing early fatigability and this finding can further be used as one of the early diagnostic tools in identifying prediabetics which is otherwise difficult in clinical scenario.
This cross-sectional study was conducted in the Department of Physiology at Rajarshi Dashrath Autonomous State Medical College, Ayodhya. The study was conducted after taking permission from the Ethics Committee of the Institution. A total of 100 prediabetic participants (n = 100) between 24–60 years old were included in the study. Prediabetics were selected from the outpatient department of the associated hospital and were screened based on the criteria for prediabetes set by the ADA according to which fasting plasma glucose levels should be between 100 mg/dL to 125 mg/dL and glycated hemoglobin (HbA1c) level should be between 5.7% to 6.4% [3].
Mosso’s ergograph was performed on the selected participants after taking a written informed consent. Proper instructions about the procedure were explained to the subjects. The subject’s forearm was fixed on the ergograph by clamps. Middle finger in the loop was pulled and index and ring fingers were fixed into metal tubes. With the middle finger extended, a 2.5 kg weight was suspended. Subject was asked to make a series of maximal contractions without moving the shoulder at regular intervals. Contractions were continued until contractions stopped. Readings were recorded in the form of a graph [8].
Development of fatigue in voluntary contracting muscle was assessed as a function of work done by the muscle in question.
Calculation of work done:
Where, W = work done (kg•m)
F = weight lifted (kg)
D = total distance moved (m)
To get the total distance moved (D), total length of all the vertical lines was measured i.e. addition of all the vertical amplitudes was done.
The subjects were asked to undergo an overnight fasting of about 10–12 h before drawing about 5 mL of blood by using standard vein puncture technique. The tubes were left at room temperature for 30 min for the blood to clot. Thereafter, serum was separated by centrifugation at 2,000 revolutions per minute (rpm) for 10 min. The LDH determination method (Selectra kit No. LDH-P SL, Reference number: LDPS 0100PS) used L-lactate as substrate buffered at 9.4 pH. LDH activity was measured as a rate reaction at 340/700 nm proportional to the amount of LDH in the sample.
An overall mean age of 42.63 ± 11.34 years with equally distributed male and female participants (n = 50 each) is shown in Table 1. The mean age of males was 44.14 ± 10.91 years while that of females was 41.12 ± 11.5 years. The overall mean LDH level was found to be 323.84 ± 26.68 unit/litre (U/L) with males having mean LDH of 333.28 ± 28.0 U/L and females with 314.4 ± 21.5 U/L. Likewise, overall work done was found to be 2.9 ± 1.2 kg•m (mean work done by males = 3.5 ± 1.4 kg•m, while females 2.39 ± 0.76 kg•m which was slightly lesser than that of males).
Mean age, serum LDH levels and work done (kg•m) by prediabetic individuals
| Parameter | Total (n = 100) | Males (n = 50) | Females (n = 50) |
|---|---|---|---|
| Age (years) | 42.63 ± 11.34 | 44.14 ± 10.91 | 41.12 ± 11.5 |
| Serum LDH (U/L) | 323.84 ± 26.68 | 333.28 ± 28.0 | 314.4 ± 21.5 |
| Work done (kg•m) | 2.9 ± 1.2 | 3.5 ± 1.4 | 2.39 ± 0.76 |
Overall distribution of male and female prediabetic individuals has been shown in Figure 1.
A correlation between work done and LDH levels in prediabetic individuals has been shown in Table 2. The results showed a strong positive correlation between LDH levels and work done (r = 0.84) which was found to be statistically significant (P < 0.05), suggesting that the higher the LDH levels, the greater the work done by the exercising muscle and consequently the lesser the fatigue.
Overall correlation of work done (kg•m) with serum LDH levels in prediabetic individuals
| Parameter | Total (n =100) | r value (Pearson’s correlation) | P value |
|---|---|---|---|
| Serum LDH (U/L) | 323.84 ± 26.68 | 0.84 | 0.047 |
| Work done (kg•m) | 2.9 ± 1.2 |
A scatter plot depiction of the correlation between serum LDH and work done by skeletal muscles in prediabetic individuals can be seen in Figure 2.
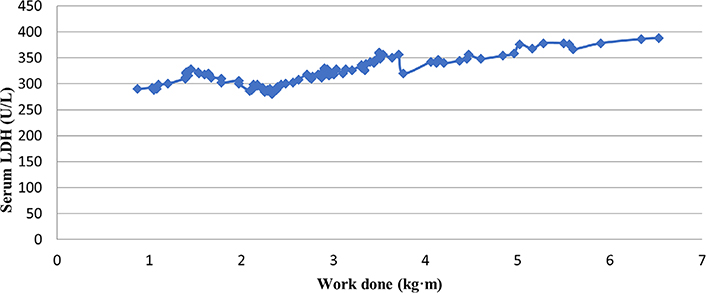
A scatter plot representation of the overall correlation of work done and serum LDH levels in prediabetic individuals
A scatter plot representation of the relation between serum LDH and work done by skeletal muscle in prediabetic males is shown in Figure 3.
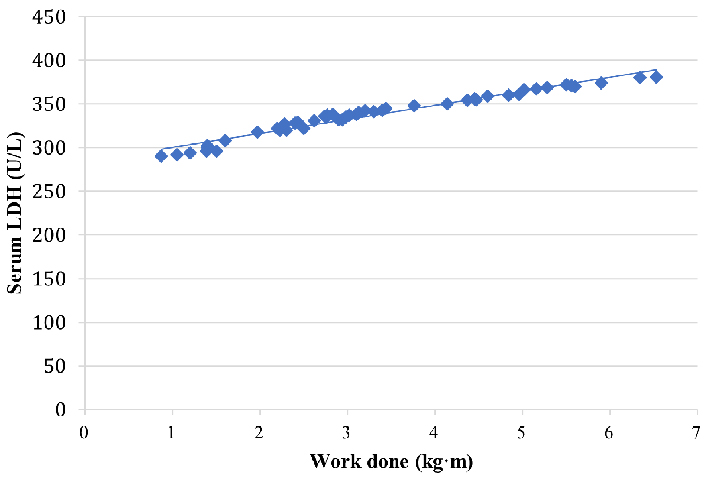
A scatter plot representation of the correlation of work done and serum LDH levels in prediabetic males
A similar scatter plot representation of the correlation of serum LDH and work done by exercising muscle in prediabetic females is shown in Figure 4.
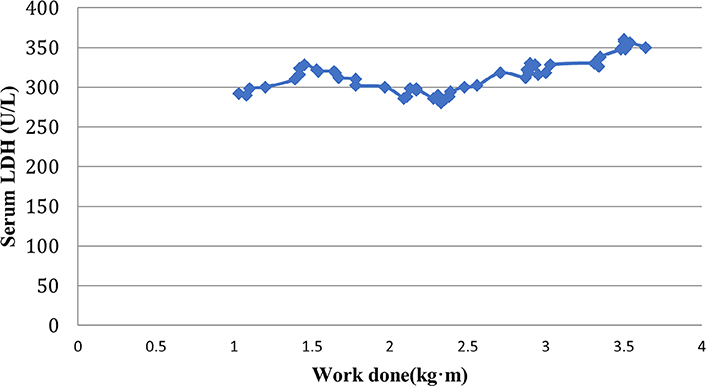
A scatter plot representation of correlation of work done and serum LDH levels in prediabetic females
A correlation between work done and LDH levels separately in prediabetic males and females is shown in Tables 3 and 4 respectively. The results showed a strong positive correlation between LDH levels and work done (r = 0.91 in males and 0.62 in females) which was found to be statistically significant (P < 0.05). The correlation was found to be stronger in males as compared to females suggesting that overall work done was greater in males than females.
A correlation of work done (kg•m) with serum LDH levels in prediabetic males
| Parameter | Total (n = 50) | r value (Pearson’s correlation) | P value |
|---|---|---|---|
| Serum LDH (U/L) | 302.61 ± 30.85 | 0.91 | 0.049 |
| Work done (kg•m) | 2.4 ± 1.48 |
A correlation of work done (kg•m) with serum LDH levels in prediabetic females
| Parameter | Total (n = 50) | r value (Pearson’s correlation) | P value |
|---|---|---|---|
| Serum LDH (U/L) | 305.14 ± 27.97 | 0.62 | 0.046 |
| Work done (kg•m) | 2.26 ± 3 |
This study aimed to assess a correlation between muscle fatigability (which was measured as a function of work done in kg•m) and serum LDH levels in prediabetic male and female subjects. The overall mean LDH levels were found to be 323.84 ± 26.68 U/L while the normal reference range is 140–280 U/L. This signifies that a raised LDH level is an adverse outcome of prediabetes which was in accordance with the study conducted by Dmour et al. [9] who found the same correlation in type 2 diabetic patients. Tanada et al. [10] in their study observed a rise in serum LDH activity with an increase in exercise which was in accordance with our study.
In general, it has been found that individuals having LDH deficiency experience easy fatigability, especially during exercise (exercise intolerance) [11]. The LDH-A subunits are specifically found in skeletal muscles and during anaerobic exercise (as in Mosso’s ergograph performed on flexors of pleximeter finger) energy to the exercising muscles is provided by breakdown of glycogen by LDH-A. If there is a deficiency of LDH-A subunit, this breakdown of glycogen does not take place and consequently energy deficit to the exercising muscle takes place resulting in fatigability. A strong positive correlation (r = 0.84) between serum LDH and work done in prediabetic individuals was found indicating that individuals showing greater work done fatigue less and hence have more serum LDH levels as compared to individuals that fatigue more with less work done. Thus, a positive correlation of work done with LDH level means that higher the work done by the exercising muscle, more will be the LDH level and less will be the fatigability and vice versa. Results showed a strong positive correlation between work done and LDH in both male and female prediabetic individuals but this correlation was stronger in case of males (r = 0.91) as compared to females (r = 0.62) suggesting that although LDH deficiency causes easy fatigability with less work done in both male and female subjects but this effect is seen more prominently in males rather than in females.
Fuchs et al. [12] and Nakamura and Schwartz [13] showed that an increase in the H+ concentration decreases the number of calcium ions bound to troponin during excitation-contraction coupling. This reduces frequency of actin myosin interactions and subsequent formation of cross bridges, thus decreasing contractile force. Moreover, Brooke and Kaiser [14] observed that the myofibrillar adenosine triphosphate activity of fast twitch fibers was more sensitive to high H+ concentrations than that of slow twitch fibers, which might be having functional implications. Thus, it might be conveniently concluded that initial impairment of muscular function during repeated fast contractions is accompanied by an initial intracellular accumulation of lactate in fast twitch fibers of exercising muscles. Concomitantly, observed differences in the activity of LDH and LDH isozymes between fiber types may influence both the formation of lactate (and H+ ions) and the transport of lactate between fiber types, thus indirectly affecting contractile ability and fatigue [15].
Determination of LDH correlation with skeletal muscle fatigue suggests strongly that LDH deficiency might be considered as an adverse outcome of prediabetes in causing early fatigability and hence LDH levels are recommended to be performed as a routine clinical test in prediabetic individuals.
LDH: lactate dehydrogenase
CM and VA contributed conception and design of the study. DS, VK and YKY organized the database. CM performed the statistical analysis. CM and VA wrote the first draft of the manuscript. SS and RKD wrote sections of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The ethical approval has been given by the Ethics Committee of Rajarshi Dashrath Autonomous State Medical College, Ayodhya, U.P.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The data cannot be shared in order to protect the privacy of the participants.
Not applicable.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
