Affiliation:
Department of Propaedeutics of Internal Diseases and Gastroenterology, A.I. Yevdokimov Moscow State University of Medicine and Dentistry, Moscow 127473, Russia
ORCID: http://orcid.org/0000-0002-6932-0729
Affiliation:
Department of Propaedeutics of Internal Diseases and Gastroenterology, A.I. Yevdokimov Moscow State University of Medicine and Dentistry, Moscow 127473, Russia
ORCID: http://orcid.org/0000-0001-6114-564X
Affiliation:
Department of Propaedeutics of Internal Diseases and Gastroenterology, A.I. Yevdokimov Moscow State University of Medicine and Dentistry, Moscow 127473, Russia
ORCID: http://orcid.org/0000-0001-9224-7382
Affiliation:
Department of Propaedeutics of Internal Diseases and Gastroenterology, A.I. Yevdokimov Moscow State University of Medicine and Dentistry, Moscow 127473, Russia
Email: vasiliy.reshetnyak@yandex.ru
ORCID: http://orcid.org/0000-0003-3614-5052
Explor Dig Dis. 2023;2:305–317 DOI: https://doi.org/10.37349/edd.2023.00033
Received: July 04, 2023 Accepted: October 31, 2023 Published: December 27, 2023
Academic Editor: Tzi-Bun Ng, The Chinese University of Hong Kong, China
The article belongs to the special issue Helicobacter Pylori and Infection: Genomics, Diagnosis, Pathogenesis, Antibiotic Resistance, Microbiota, Cancer, Prevention and Therapeutics
Aim: This study aims to determine the significance of chronic hyperglycemia for the reduced efficacy of eradication therapy in patients with type 2 diabetes mellitus (T2DM) and Helicobacter pylori (H. pylori)-associated upper gastrointestinal tract pathology as well as for H. pylori survival.
Methods: A prospective randomized study with the participation of 180 patients (87 men and 93 women) with H. pylori-associated upper gastrointestinal pathology was carried out. Ninety of these patients were with T2DM and 90 were without diabetes mellitus (DM). The patients were divided into 4 groups of 45 patients: the group 1 included non-diabetic patients treated with the classical triple eradication scheme; the group 2 included patients with T2DM treated with the classical triple eradication scheme; the group 3 included non-diabetic patients treated with bismuth quadro-therapy; the group 4 included patients with T2DM treated with bismuth quadro-therapy. The presence of H. pylori and evaluation of eradication efficacy was carried out using the Helix breath test.
Results: The effectiveness of 1st line anti-Helicobacter therapy was higher (88.2%) in patients without diabetes in comparison with the group of patients with concomitant T2DM (74.7%). The efficacy of classical triple eradication therapy in patients with concomitant T2DM was 69.1%, and quadro-therapy was 80.5%. There was significantly lower effectiveness (P < 0.017) of eradication therapy in patients with T2DM and glycated hemoglobin (HbA1c) level ≥ 7.0% as compared with the group of patients in whom the target (≤ 6.5%) level of HbA1c was achieved.
Conclusions: Chronic hyperglycemia has a favorable effect on the viability of H. pylori bacteria in patients with T2DM. A hypothesis explaining the reduced efficacy of eradication therapy in patients with hyperglycemia has been proposed.
The number of patients with type 2 diabetes mellitus (T2DM) in the world has more than doubled in the past 10 years, and by the end of 2021, it exceeded 537 million people [1]. The International Diabetes Federation predicts that 643 million people will have diabetes mellitus (DM) by 2030 and 783 million by 2045 [1]. In the Russian Federation, as in all countries of the world, there is a significant increase in the prevalence of DM. According to the Federal Register of DM in the Russian Federation as of January 01, 2023, there were 4,962,762 persons (3.42% of the population) on the dispensary registry, of which: 92.3% (4,581,990) had T2DM, 5.6% (277,092) had type 1 DM, and 2.1% (103,680) had other types of DM, including 8,758 women with gestational DM [1]. However, these data underestimate the real number of patients, because they only take into account identified and registered cases of the disease.
Helicobacter pylori (H. pylori) has been shown to be detected more frequently in patients with DM than in the general population [2–4]. According to a meta-analysis that summarized the results of 79 studies (57,397 people), patients with concomitant DM had a higher prevalence of H. pylori infection than patients without diabetes [odds ratio (OR) 2.05, P < 0.01] [5, 6]. In patients with diabetes, the risk of developing H. pylori-associated diseases is significantly increased [2, 7–9].
The relationship between H. pylori infection and the risk of developing T2DM remains controversial and ambiguous. At the same time, many papers present data indicating the low effectiveness of eradication therapy for H. pylori infection in patients with diabetes compared with patients without this disease [10, 11, 12]. Studies performed on various patient populations lead to a consensus that DM significantly reduces the likelihood of success of anti-Helicobacter therapy. Mkrtumyan et al. [2] compared the effectiveness of classical triple eradication therapy (amoxicillin 1,000 mg 2 times daily, omeprazole 20 mg 2 times daily, clarithromycin 500 mg 2 times daily), duration of 7 days in patients with concomitant T2DM and patients without diabetes [2]. A significantly lower efficacy was shown in the group of patients with concomitant T2DM compared with the group of patients without diabetes (50% and 85%, respectively, P < 0.05) [2]. In a study by Gasbarrini et al. [13], a study of the effectiveness of the classical triple eradication treatment regimen in patients with concomitant DM, showed that the level of eradication efficiency in them is lower than in patients without this pathology (65% vs. 92%, P < 0.05). Andreev et al. [14], showed that in the group of patients with successful anti-Helicobacter therapy, there were 12% of patients with concomitant T2DM, while in the group of patients who did not achieve eradication, there were 38.9% of patients with this disease, OR 0.21 [95% confidence interval (CI): 0.06–0.69, P = 0.0102]. Horikawa et al. [15], published a meta-analysis of 8 studies (n = 693 patients, including 273 with concomitant DM) in which they showed that the relative risk (RR) of inadequate eradication was 2.19 (95% CI: 1.65–2.90, P < 0.001) in patients with DM compared with patients without diabetes.
A correlation has been shown between a higher frequency of H. pylori detection in DM patients and a lower efficiency of eradication therapy in them, depending on the level of chronic hyperglycemia [3]. The mechanisms that link chronic hyperglycemia with an increased incidence of H. pylori, as well as the mechanisms that contribute to improved survival of this bacterium in patients with T2DM during eradication measures, remain unknown.
The aim of this work is to determine the significance of chronic hyperglycemia for the reduced efficacy of eradication therapy in patients with T2DM and H. pylori-associated upper gastrointestinal tract pathology as well as for H. pylori survival.
We conducted a prospective randomized study in which we examined 180 patients with H. pylori-associated pathology of the upper gastrointestinal tract, including 90 patients with T2DM and 90 patients without DM. A prospective randomized study was conducted, which examined 180 patients with H. pylori-associated pathology of the upper gastrointestinal tract, including 90 patients with T2DM and 90 patients without DM. The study included 87 men (48.3%) and 93 women (51.7%) in whom erosive gastritis was revealed in 57.8%, duodenal ulcer in 31.1%, and peptic ulcer in 11.1% of patients. The patients were treated in 2 therapeutic and gastroenterological departments of the main clinical hospital of the Ministry of Internal Affairs of Russia from September 2018 to April 2021. The mean age of the patients was 48.35 ± 5.25 years.
• Female or male patients between 18 years and 70 years of age who have been diagnosed with H. pylori-associated upper gastrointestinal tract pathology with or without concomitant T2DM.
• Informed consent signed by the patient.
• Pregnancy.
• Breast-feeding.
• Established allergic reactions to drugs from the planned eradication therapy.
• Use of proton pump inhibitors (PPIs) and/or antibacterial drugs within 4 weeks before the study.
• Lack of confirmation of H. pylori infection by the HELIK-test.
• Established psychiatric (endogenous) disorders.
• Concomitant severe gastrointestinal tract pathology or complications of digestive diseases.
• Severe comorbidities (decompensated cardiovascular disease, lung disease with signs of respiratory failure, chronic kidney disease stage 4–5, Child-Pugh stage C cirrhosis).
• Combined intake of gastro- and hepatotoxic drugs [statins, non-steroidal anti-inflammatory drugs (NSAIDs), cytostatics, antifungal agents, anti-tuberculosis drugs, sulfonamides, anti-gout, hormonal drugs].
• Refusal or inability to sign the study participant’s informed consent.
• The development of side effects requiring immediate discontinuation of treatment (anaphylactic shock, Quincke’s edema, development of pseudomembranous colitis).
• Alcohol consumption during treatment.
• Non-compliance with the treatment regimen.
• Withdrawal of patient consent.
• Failure to appear at the control visit.
• Violation of dosage and frequency of medication administration.
• Patients who have had a moderate to severe new coronavirus infection.
The patients were divided into four groups depending on the ongoing anti-Helicobacter therapy according to the Maastricht V consensus (2015) and the recommendations of the Russian Gastroenterological Association (2018) and the presence of DM:
• Group 1 (45 patients). Patients with H. pylori-associated gastric and duodenal diseases and without DM who were treated with anti-Helicobacter therapy using a triple scheme: omeprazole (20 mg 2 times daily), amoxicillin (1,000 mg 2 times daily) and clarithromycin (500 mg 2 times daily) for 14 days.
• Group 2 (45 patients). Patients with H. pylori-associated gastric and duodenal diseases and T2DM who were treated with anti-Helicobacter therapy using a triple scheme: omeprazole (20 mg 2 times daily), amoxicillin (1,000 mg 2 times daily) and clarithromycin (500 mg twice daily) for 14 days.
• Group 3 (45 patients). Patients with H. pylori-associated gastric and duodenal diseases and without DM who were treated with anti-Helicobacter therapy using quadro-therapy: omeprazole (20 mg 2 times daily), bismuth tripotassium dicitrate (120 mg 4 times daily), tetracycline (500 mg 4 times daily), metronidazole (500 mg 3 times daily) for 14 days.
• Group 4 (45 patients). Patients with H. pylori-associated gastric and duodenal diseases and T2DM who were treated with anti-Helicobacter therapy using quadro-therapy: omeprazole (20 mg 2 times daily), bismuth tripotassium dicitrate (120 mg 4 times daily), tetracycline (500 mg 4 times daily), metronidazole (500 mg 3 times daily) for 14 days.
Patients with T2DM included in groups 2 and 4 were additionally divided into subgroups based on the level of glycated hemoglobin (HbA1c):
• Group 2: patients with HbA1c levels ≤ 6.5%, 31 patients; patients with HbA1c levels ≥ 7.0%, 14 patients.
• Group 4: patients with HbA1c levels ≤ 6.5%, 35 patients; patients with HbA1c levels ≥ 7.0%, 10 patients.
There were no statistically significant differences (P > 0.05) when analyzing the groups with respect to gender and age.
All patients underwent clinical and laboratory examinations, including physical examination, general clinical examination (automatic hematological analyzer Advia® 2120i, Siemens, Germany) and biochemical examination (biochemical analyzer Siemens Dimension® Xpand® Plus, Siemens, Germany) blood tests, fibrogastroduodenoscopy (fibrogastroduodenoscope GIF-H180J, Olympus, Japan) to detect H. pylori-associated pathology of the stomach and duodenum, diagnosis of H. pylori infection using non-invasive breath test (HELIK-test, Association of Medicine and Analytics, Russia), ultrasound examination (ultrasound device HI VISION Ascendus, Hitachi, Japan) of abdominal organs to assess the condition of liver, spleen, pancreas, and gallbladder, and presence of concomitant pathology of these organs. Eradication efficacy was assessed using HELIK-test 4 weeks after completion of anti-Helicobacter therapy.
Patients with T2DM additionally had capillary blood glucose levels determined using a glucometer (Satellite Express PKG-03, Elta, Russia) and strips (Satellite Express, Elta, Russia) during the period: the day before, on the 7th, 14th day, and in 4 weeks after completion of the course of anti-Helicobacter therapy. Also, patients with DM had HbA1c levels determined on an empty stomach on a biochemical analyzer (Siemens Dimension Xpand Plus, Siemens, Germany) before eradication therapy and 4 weeks after completion of the course of anti-Helicobacter therapy.
Patients with concomitant T2DM received the following groups of hypoglycemic drugs: biguanides (metformin) at a dosage of 850–2,000 mg/day, depending on the level of glucose and HbA1c, dipeptidyl peptidase-4 inhibitors (iDPP-4) at a dosage of 100 mg/day, sodium-glucose cotransporter type 2 inhibitors (iSGLT-2) 10 mg/day, sulfonylurea derivatives at a dosage of 2–4 mg/day (Table 1).
The number of patients with T2DM, depending on the hypoglycemic therapy taken
| Group | Number of patients treated with hypoglycemic drugs | ||||
|---|---|---|---|---|---|
| Biguanides | Biguanides + iDPP-4 | Biguanides + iSGLT-2 | Biguanides + sulfonylurea derivatives | Biguanides + iDPP-4 + sulfonylurea derivatives | |
| 2 | 25 | 6 | 4 | 5 | 5 |
| 4 | 29 | 2 | 6 | 6 | 2 |
The correlation between factor and performance characteristics was not statistically significant (P > 0.05). Thus, there was no significant difference between the groups in the intake of hypoglycemic therapy.
The data were statistically processed using the MedCalc program package, version 20.013 (Belgium) in the Microsoft Windows 10 environment (USA), using the principles of mathematical analysis of medical and biological research and according to the requirements for medical data processing [16]. Arithmetic mean (X) value and standard deviation (σ) were used for the obtained results, whose distribution in the variation series was normal. Student’s t-test was used to assess intergroup differences. Pearson’s chi-square test (χ2) was used to test the hypothesis about the regularity of observed differences. Indicators were considered statistically significant when the probability of error was P < 0.05.
The study protocol was approved by the Interuniversity Ethics Committee. Patients participating in the study were explained in detail and fully about the study. All patients signed voluntary informed consent to participate in this study.
The distribution of patients in the groups by nosological forms of H. pylori-associated pathology is shown in Table 2.
The number of patients in groups with various H. pylori-associated pathologies of the upper gastrointestinal tract
| Group | Number of patients (n) in groups with various H. pylori-associated pathologies | ||
|---|---|---|---|
| Erosive gastritis | Duodenal ulcer | Gastric ulcer | |
| 1 | 25 | 15 | 5 |
| 2 | 30 | 13 | 2 |
| 3 | 20 | 18 | 7 |
| 4 | 29 | 10 | 6 |
| Total | 104 | 56 | 20 |
The diagnosis was established on the basis of patients’ complaints, anamnesis, physical examination, and endoscopic findings. The main complaints of patients were: discomfort, heaviness and/or pain in the epigastric region, heartburn, nausea, sour belching, vomiting, feeling of early satiety, loss of appetite, constipation, diarrhea, and flatulence. Patients with T2DM also had complaints of dry mouth, thirst, and frequent urination.
As a result of the treatment, healing of erosions and ulcers was observed in patients in all groups.
The number of patients who fully complied with the study protocol was 168: 43 patients in the first group, 42 each in the second and third groups, and 41 in the fourth group. Three out of seven patients with T2DM did not complete treatment due to the development of adverse events in response to the ongoing therapy, and 4 were excluded from the study due to failure to attend the control appointment 14 days after anti-Helicobacter therapy. Four patients from groups 1 and 3 failed to appear for the control appointment, 1 person from group 3 stopped taking the prescribed eradication therapy on his own, and therefore these patients were not included in the final result.
The effectiveness of first-line eradication therapy in all patients included in the study without DM was 88.2% (75 patients out of 85 patients). In all investigated patients with T2DM, the efficacy of anti-Helicobacter therapy was 74.7% (62 patients out of 83) and was significantly lower (OR 0.3937, 95% CI: 0.1725–0.8981, P = 0.0267), than in patients without T2DM (88.2%).
Triple and quadro-therapy efficacy was shown in the study groups (Table 3). The effectiveness of quadro-therapy in patients without DM (third group) was 92.9% (39 patients out of 42) and was higher than in patients of the first group (also without DM) who received a triple regimen of anti-Helicobacter therapy: 83.7% (36 patients out of 43).
Efficacy of 1st line eradication therapy in the groups
| Groups | Number of patients with effective anti-Helicobacter therapy | Number of patients receiving anti-Helicobacter therapy in groups | % |
|---|---|---|---|
| 1 | 36 | 43 | 83.7 |
| 2 | 29 | 42 | 69.1 |
| 3 | 39 | 42 | 92.9 |
| 4 | 33 | 41 | 80.5 |
At the same time, the efficacy of quadro-therapy in patients with T2DM (fourth group) was 80.5% (33 patients out of 41), which was higher than in patients with T2DM of the second group who received triple anti-Helicobacter therapy: 69.1% (29 patients out of 42). The presence of T2DM significantly reduces the efficacy of eradication therapy both in three-component therapy and quadro-therapy. There were no significant differences between the groups receiving triple anti-Helicobacter therapy (groups 1 and 2) and quadro-therapy (groups 3 and 4, P > 0.05).
The following side effects were noted in patients taking anti-Helicobacter therapy: diarrhea, nausea, vomiting, dysgeusia, rash, lack of appetite, headache, and insomnia. The incidence of adverse events when using 1st line eradication therapy in different groups was:
• in patients without diabetes who received the triple regimen 26.7%;
• in patients who received triple therapy with concomitant DM 22.2%;
• in patients who received quadro-therapy without diabetes 28.9%;
• in patients with concomitant type 2 diabetes taking quadro-therapy 31.1%.
Patients receiving quadro-therapy with bismuth preparations had a slightly higher incidence of adverse events than patients receiving triple therapy, but no significant differences were observed.
To evaluate the effectiveness of the received hypoglycemic therapy, HbA1c was determined in all patients with T2DM over time. Depending on the achievement of the target level of HbA1c in patients with T2DM who received triple (group 2) and quadro (group 4) anti-Helicobacter therapy, an analysis of the effectiveness of eradication therapy was carried out (Table 4).
The number of T2DM patients according to HbA1c level
| Level of HbA1c | Number of patients in groups depending on HbA1c level | |
|---|---|---|
| Group 2 | Group 4 | |
| ≤ 6.5% | 29 | 33 |
| ≥ 7.0% | 13 | 8 |
In 62 (74.7%) out of 83 patients with T2DM, the HbA1c level was ≤ 6.5%, and in 21 (25.3%) patients, the target HbA1c level was not reached, and it was ≥ 7.0%. The data suggest that a quarter of patients with T2DM, despite taking hypoglycemic drugs, maintained a high level of glycemia for at least 2–3 months. There were no significant differences between the groups receiving three-component anti-Helicobacter therapy (group 2) and quadro-therapy (group 4) in the number of patients who achieved and did not reach the target indicator for HbA1c.
The content of fasting glucose in capillary blood in the studied patients with T2DM and HbA1c levels ≤ 6.5% was within the normal range and amounted to less than 5.0 mmol/L immediately after, as well as on days 7 and 14 after eradication therapy. On the contrary, in the group of patients with T2DM and HbA1c level ≥ 7.0%, fasting glucose in capillary blood was elevated and amounted to more than 7.0 mmol/L both after and on days 7, 14 after anti-H. pylori therapy.
The effectiveness of anti-Helicobacter therapy in patients with T2DM, depending on the HbA1c levels is shown in Figure 1.
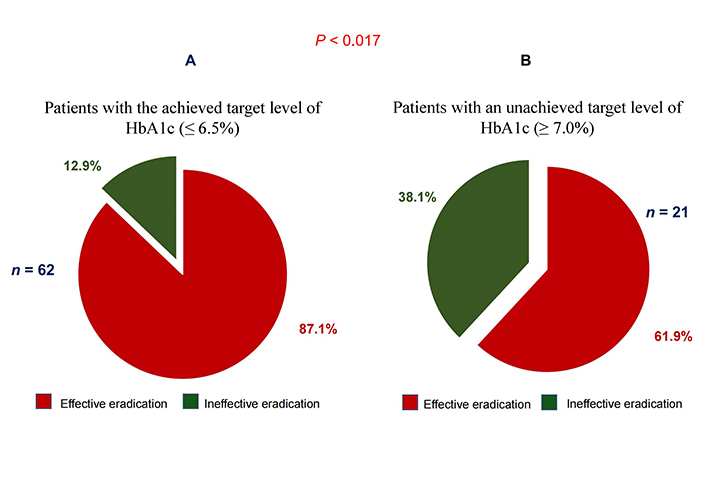
Eradication efficacy in T2DM patients with HbA1c level ≤ 6.5% (A) and HbA1c level ≥ 7.0% (B)
Effective eradication of H. pylori was observed in 54 (87.1%) of 62 patients with T2DM and HbA1c level ≤ 6.5% (Figure 1A). Whereas in patients with HbA1c levels ≥ 7.0%, effective eradication of H. pylori was observed in only 61.9% (13 of 21) of patients (Figure 1B). There was significantly (P < 0.017) lower efficacy of eradication therapy in patients with T2DM and HbA1c levels ≥ 7.0% compared with the group of patients in whom the target (≤ 6.5%) HbA1c level was achieved.
H. pylori occurs in almost half of the world’s population [17, 18]. H. pylori is a bacterium that is often found in patients with chronic gastritis, peptic ulcer, and gastric cancer, as well as among the healthy population [19, 20]. Over a 40-year period of study of this bacterium, it has been convincingly shown that in patients with DM, both of the first and second types, H. pylori is detected more frequently than in the general population [2, 3, 21, 22]. The high incidence of H. pylori in patients with DM is accompanied by low efficacy of eradicate on therapy [10, 11]. The data presented in this study also indicate that in patients with T2DM, the effectiveness of anti-Helicobacter therapy was significantly lower (OR 0.3937, 95% CI: 0.1725–0.8981, P = 0.0267) than in patients without DM. Moreover, the use of standard treatment regimens for patients with H. pylori infection, according to the Maastricht VI consensus of 2022 [19] and the recommendations of the Russian Gastroenterological Association of 2018 [23] as first-line therapy, showed that the effectiveness of quadro-therapy with bismuth drugs was higher than three-component anti-Helicobacter therapy. However, the efficacy of both quadro-therapy and three-component H. pylori eradication scheme in patients with T2DM remains lower than in patients without DM.
The precise mechanisms that contribute to improved H. pylori survival in patients with DM during eradication measures remain undeciphered. However, it is well known that one of the important signs of DM is hyperglycemia. Domestic and foreign literature data indicate that there is a direct correlation between the level of hyperglycemia and lower eradication efficacy in patients with DM [3, 24, 25]. At the same time, it is known that chronic hyperglycemia leads to increased susceptibility of DM patients to many infectious agents [3, 26]. However, there is still no understanding of how chronic hyperglycemia and the maintenance of H. pylori metabolic homeostasis in patients with DM are related. The disclosure of the mechanisms of this relationship is of great scientific and practical importance.
It is well known that for long-term colonization of a habitat, the bacterium must adapt to the nutritional conditions. For the vital activity, growth, and reproduction of H. pylori in the gastric mucosa, the bacterium uses amino acids and carboxylic acids [27, 28]. At the same time, H. pylori is practically independent of carbohydrates, such as glucose [27, 28]. However, research conducted in the 1990s of the last century and later, indicates that H. pylori has enzyme systems capable of utilizing D-glucose [29, 30]. There exists experimental evidence indicating that the addition of glucose to the nutrient medium during the cultivation of H. pylori enhances its growth [25]. On this basis, it follows that the viability and reproduction of bacteria may depend not only on the presence of amino acids and carboxylic acids, but also on the presence of glucose in its environment. Normally, there is no glucose in the stomach. That is why H. pylori has adapted to use the amino acids and carboxylic acids produced in the stomach as a result of the breakdown of food proteins as an energy and plastic material.
T2DM is often accompanied by the development of chronic hyperglycemia. Persistent and prolonged increases in plasma glucose in patients with T2DM trigger compensatory mechanisms aimed at normalizing blood glucose levels. As a result, the gastric mucosa begins to perform an excretory function for glucose, which can lead to its accumulation in the supraepithelial mucus layer. This leads to the fact that H. pylori in patients with T2DM and chronic hyperglycemia receive an additional source of nutrition. And if so, the higher frequency of H. pylori detection in T2DM patients than in the general population could be explained by chronic hyperglycemia.
Normalization of serum glucose levels in T2DM patients, due to the correct selection and administration of hypoglycemic drugs, leads to the elimination of chronic hyperglycemia and depriving H. pylori of additional nutrition. This fact may be of particular importance during anti-Helicobacter therapy. H. pylori eradication regimens contain antibacterial drugs (clarithromycin, metronidazole, bismuth preparations, etc.) and drugs that reduce the production of hydrochloric acid. The use of antisecretory drugs is aimed at creating optimal conditions for acid-dependent antibacterial agents [31, 32]. Application of antisecretory drugs has a double effect on H. pylori with the opposite effect: increase of pH in the stomach favorably affects the vital activity of H. pylori; and decrease of protein hydrolysis rate leads to deprivation of H. pylori of nutrients. Lack of nutrients and the intake of antibacterial agents by patients lead either to death of the microorganism or to its transition to a dormant form [33]. The second occurs rarely with powerful antibacterial therapy. This is how the process of bacterial eradication occurs in patients without DM or in patients with T2DM with adequately selected hypoglycemic therapy.
In case of ineffectiveness of hypoglycemic drugs and chronic hyperglycemia persists in T2DM patients, who undergo anti-Helicobacter therapy, amino acid deficiency develops. This leads to the fact that H. pylori, due to the lack of nutritional ingredients, can and begins to use glucose as an energy and plastic material. It is likely that this mechanism allows the microorganism to successfully survive the extreme conditions of eradication, which leads to a decrease in the effectiveness of anti-Helicobacter therapy.
Chronic hyperglycemia can be assessed using the level of HbA1c in the blood. The indicator plays an important role in monitoring the dynamics of blood glucose levels in patients with DM and in assessing the effectiveness of taking hypoglycemic drugs [34]. In 2011, the World Health Organization officially recommended HbA1c values ≥ 6.5% as a diagnostic cut-off point for DM [35]. This indicator reflects the integrated blood glucose level over the past three to four months [36].
The data presented in our study on the effectiveness of anti-Helicobacter therapy in T2DM patients with HbA1c levels ≤ 6.5% indicate that H. pylori eradication under these conditions is more effective than in patients with T2DM and HbA1c levels ≥ 7.0%. In 21 patients out of 83 examined T2DM patients, HbA1c level ≥ 7.0% did not reach the target level and remained elevated. This means that despite taking hypoglycemic medications, hyperglycemia persisted in these patients for at least two to three months. It was in these patients who did not reach the target HbA1c level that there was a significantly (P < 0.017) lower efficiency of eradication therapy compared with the group of patients who achieved the target level (≤ 6.5%) of HbA1c (Figure 1). These data indirectly confirm the above assumption and indicate that chronic hyperglycemia in T2DM against the background of developing amino acid deficiency in the stomach associated with the use of PPIs during anti-H. pylori therapy leads to the fact that H. pylori begins to use glucose as an energy and plastic material. This creates a more favorable environment for the survival of H. pylori bacteria under extreme conditions of eradication.
This assumption is confirmed by the data of Tseng [37] who showed that the use of insulin to normalize blood glucose levels in T2DM patients significantly increases the frequency of H. pylori eradication compared to patients with DM without insulin. The higher efficacy of H. pylori eradication in T2DM patients when using insulin suggests that these patients have normalization of blood glucose levels during insulin therapy. The mucous membrane of the digestive system, with the normalization of blood glucose levels, is switched off from the excretion of glucose from the body. Amino acid deficiency that develops while taking PPIs and the absence of glucose in the supraepithelial layer of mucus, due to the normalization of blood glucose levels, leads to an increase in the efficiency of H. pylori eradication.
Separate studies report that H. pylori is not associated with either insulin resistance or the prevalence of T2DM [38, 39]. The likelihood of such a conclusion is most probably associated with the inclusion in the study of T2DM patients without significant chronic hyperglycemia or with well-corrected hypoglycemic therapy.
If the assumption about the use of glucose by the H. pylori bacterium as an additional energy and plastic material in conditions of chronic hyperglycemia is correct, then before conducting eradication H. pylori in T2DM patients, it is necessary to monitor and purposefully correct the level of blood glucose and HbA1c according to the algorithm shown in scheme (Figure 2).
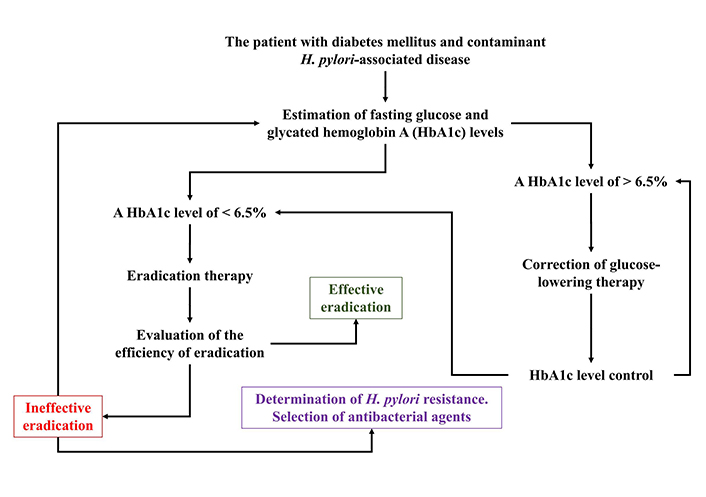
Algorithm for monitoring and targeted correction of the HbA1c level in patients with T2DM and H. pylori-associated diseases
Note. Reproduced from “Maintaining the metabolic homeostasis of Helicobacter pylori through chronic hyperglycemia in diabetes mellitus: a hypothesis,” by Reshetnyak VI, Maev IV. World J Meta-Anal. 2022;10:238–43 (https://www.wjgnet.com/2308-3840/full/v10/i5/238.htm). CC BY-NC.
The algorithm has important practical value both for monitoring the level of glycemia in dynamics against the background of taking hypoglycemic drugs and their effectiveness, and for achievement of success of eradication therapy in T2DM patients and with H. pylori-associated diseases.
It is also worth considering the relationship between DM and infections parasitic (IPs). In a systematic review and meta-analysis by Zibaei et al. [40], which examined 29 studies, the pooled prevalence of IPs in patients with diabetes was 26.5% (95% CI: 21.8–31.7%), with heterogeneity I2 = 93.24%; P < 0.001. The highest prevalence by geographic region was observed in the Americas [13.3% (95% CI: 9.6–18.0)]. There was a significant association between the prevalence of intestinal parasites in diabetic patients compared with controls (OR 1.72, 95% CI: 1.06–2.78). However, this topic requires further research.
In conclusion, a prospective randomized study was conducted, which showed a lower efficacy of eradication therapy in patients with T2DM and H. pylori-associated disease compared with patients without diabetes, which coincides with the literature data presented in domestic and foreign scientific articles. The results of the study showed that four-component therapy with bismuth preparations has a higher efficacy than triple anti-Helicobacter therapy. The data obtained, reveal that the effectiveness of quadro-therapy and a three-component H. pylori eradication scheme in patients with T2DM is lower than in patients without DM. The correlation between the low effectiveness of anti-Helicobacter therapy and chronic hyperglycemia in patients with T2DM and H. pylori-associated diseases was noted. It has been shown that the normalization of blood glucose levels in patients with T2DM neutralizes this relationship.
Analysis of our data and academic data allowed us to suggest a positive effect of chronic hyperglycemia on the viability and reproduction of H. pylori in patients with T2DM. Available data suggest that in T2DM patients and the presence of chronic hyperglycemia, the H. pylori bacterium uses both amino acids and glucose for its vital activity. A hypothetical mechanism has been put forward to explain the relationship between chronic hyperglycemia and changes in H. pylori metabolism, which lead to the creation of more favorable conditions for life and reproduction of the microorganism in patients with T2DM and allows microorganisms to survive in extreme conditions of eradication. The hypothesis explains the high incidence of H. pylori detection in T2DM patients, as well as the lower effectiveness of eradication therapy in them. The hypothesis requires further confirmation using various molecular methods of research, which will be important for the success of eradication therapy.
An algorithm for monitoring and targeted correction of HbA1c levels in T2DM patients and H. pylori-associated diseases during eradication therapy has been proposed.
CI: confidence interval
DM: diabetes mellitus
H. pylori: Helicobacter pylori
HbA1c: glycated hemoglobin
iDPP-4: dipeptidyl peptidase-4 inhibitors
OR: odds ratio
PPIs: proton pump inhibitors
T2DM: type 2 diabetes mellitus
LGB, IVM, DTD, and VIR have equally contributed to Conceptualization, Formal Analysis, Writing—original draft, Writing—review & editing. All authors read and approved the final version.
The authors declare that they have no conflicts of interest.
The study “The significance of chronic hyperglycemia for the reduced efficacy of eradication therapy in patients with type 2 diabetes mellitus and for Helicobacter pylori survival” was approved by the Interuniversity Ethics Committee.
The informed consent to participate in the study was obtained from all participants.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Elias Kouroumalis ... Argyro Voumvouraki
Rosabel Corrales ... Rafael Llanes
Natalia V. Baryshnikova ... Alexander N. Suvorov
Tatyana Anatolievna Kuchmenko ... Arina Kopaeva
Surbhi Dumra, Abhishek Ray
