Affiliation:
1Arud Centre for Addiction Medicine, 8001 Zürich, Switzerland
†These authors share the first authorship.
ORCID: https://orcid.org/0000-0001-6046-1771
Affiliation:
2Swisstransplant, CH-3011 Berne, Switzerland
†These authors share the first authorship.
Affiliation:
1Arud Centre for Addiction Medicine, 8001 Zürich, Switzerland
3Institute of Primary Care, University Hospital Zurich, 8091 Zürich, Switzerland
#These authors share the last authorship.
Email: p.bruggmann@arud.ch
ORCID: https://orcid.org/0000-0002-0436-8895
Explor Dig Dis. 2023;2:297–304 DOI: https://doi.org/10.37349/edd.2023.00032
Received: August 05, 2023 Accepted: October 23, 2023 Published: December 25, 2023
Academic Editor: Maria Carlota Londoño, University of Barcelona, Spain
Aim: In Switzerland, the first access to interferon-free direct-acting antivirals (DAAs) for hepatitis C virus (HCV) treatment was in 2014. This study aimed to analyze the effects of DAAs on the yearly listed numbers of HCV RNA-positive (RNA+) patients and their mortality on the Swiss organ transplantation waiting list (SOWL).
Methods: In this retrospective secondary time series analysis of yearly aggregated data on listed and delisted patients from a subset of HCV RNA+ patients on the SOWL, listed patients were grouped by the requested organ, and delisted patients by reason. Time series were split into two periods of equal length, the phases before and after DAA implementation, and the mean difference was tested using the Mann-Whitney U test.
Results: From 2008 to 2019, 328 HCV RNA+ patients were listed on SOWL, 86.6% requesting liver, 11.6% kidney, and 1.8% other organ transplantations. A total of 285 RNA+ patients were delisted from SOWL: 14.7% died, 75.4% had been transplanted, and 9.8% were delisted without surgery. There were significant reductions of patients listed for requesting any organ (–21.7, P = 0.004), liver (–18.3, P = 0.004), or kidney (–3.0, P = 0.031) comparing the periods before and after DAA launch. The mean number of delistings after transplantation (–11.2, P = 0.010), or death (–4, P < 0.001) show a significant reduction.
Conclusions: With DAAs, the rising trend of HCV RNA+ people waiting for organs was broken, as was the increasing trend of mortality on the SOWL among HCV RNA+ individuals.
The prevalence of chronic hepatitis C in Switzerland in 2020 was estimated to be 0.37%, corresponding to 32,100 people carrying the virus [1]. Approximately 40% of those affected in Switzerland are undiagnosed [1]. The goal of both the Swiss Hepatitis Strategy and the World Health Organization (WHO) global strategy is to eliminate hepatitis C by 2030 [2, 3].
The sequelae of hepatitis C virus (HCV) infection include the development of liver cirrhosis within 20 years in 20% of those affected [4]. Primary hepatocellular carcinoma (HCC) is developed by 2–4% of all chronically infected patients [4]. HCV-related mortality was substantial at the last survey, with approximately 200 deaths per year, and five times higher than that of human immunodeficiency virus (HIV) [5]. Chronic kidney disease is frequently linked to HCV infection and may even result from it [6]. Hepatitis C infection is systemic and a risk factor for numerous serious extrahepatic manifestations such as renal disease, cardiovascular events, diabetes mellitus, and malignant lymphoma [7]. Patients with kidney disease who also have HCV may face serious complications, including a higher risk of mortality for those with end-stage renal disease, treating HCV effectively in this scenario can help improve these adverse outcomes [6]. HCV prevalence among kidney transplant recipients varies between 5–50%, mainly due to the HCV-transmission risk during hemodialysis [8].
Since the launch of new direct-acting antivirals (DAAs) in 2014, the cure rate has been 95% [9]. Previously, HCV therapy consisted of interferon-containing combinations with significant side effects, several contraindications, and comparatively modest cure rates [10]. Timely treatment of hepatitis C before the onset of advanced liver fibrosis prevents the development of liver cirrhosis or HCC. Early therapy can also prevent the occurrence of extrahepatic manifestations [11, 12]. HCV treatment of patients with cirrhosis provides the opportunity to stop the progression of liver decompensation and decrease the risk of HCC [13].
The allocation of a donor organ in Switzerland is controlled and carried out at a national level via an electronic system operated by the Federal Office of Public Health (FOPH), the Swiss Organ Allocation System (SOAS) [14]. The national allocation body Swisstransplant uses SOAS and is responsible for maintaining the nationwide Swiss organ transplantation waiting list (SOWL), allocating organs, coordinating exchanges with foreign allocation organizations, and organizing and coordinating all allocation-related activities [14].
The aim of this study was to examine the effects of the release of interferon-free DAAs for HCV treatment in the summer 2014 on the yearly numbers of listed patients, grouped by requested organ (any organ, liver, kidney), and of the delisted patients, grouped by reason (transplanted, not transplanted, deceased). As a second objective, we compared the growth dynamics of the time series between the two periods for each indicator.
This study was a retrospective secondary time series analysis of yearly aggregated data from a subset of HCV RNA-positive (RNA+) patients on the SOWL for the 12-years period from 2008 to 2019 (n = 328, 3.9% of all patients) [15].
We split the time series into two periods of equal length: period 1 (P1: 2008–2013), the phase before the implementation of DAA medications; and period 2 (P2: 2014–2019), the phase thereafter, and tested the mean difference P2 – P1 [subtraction of the respective measure for P1 from the respective measure for P2 (sum, mean)] using the Mann-Whitney U test.
In addition, we calculated linear and exponential curve estimations [equations: Yt = b0 + b1 × t, Yt = b0 × e (b1 × t), respectively; Where b0 is the constant term, b1 is the regression parameter, e is the base of the natural logarithm (Euler’s number), and t from 1 to 6 counts the number of years within the period]. If the analyses of variance (ANOVA) for both regression models yielded significant results, we used the estimation with a higher coefficient of determination R square (R2). All statistical tests were performed using a two-sided 95% alpha level. The calculations were performed using IBM SPSS Version 29.
During the 12-year observation period from 2008 to 2019, 328 HCV RNA+ patients (73% male, 27% female; 50.6% in the birth cohort from 1960 to 1969) were listed in the SOWL. These cases comprised 284 (86.6%) requesting a liver, 38 (11.6%) a kidney, and 6 (1.8%) other organ transplantations (1 heart, 5 pancreas). During the same period, 285 patients were excluded from SOWL. Of these, 42 (14.7%) died, while 243 (85.3%) survived, either with transplantation (215/243, 88.5%) or without such surgery (28/243, 11.5%).
HCV RNA+ patients on the SOWL during the 6-year period before the introduction of DAAs are compared in Table 1. It shows the sum, mean, and standard deviations (SD) for P1 (2008–2013), P2 (2014–2019), and P2 – P1, together with the significance of the respective Mann-Whitney U test. The overall trends of the SOWL (2008–2022) irrespective of HCV-status are reported in the Supplementary material.
HCV RNA+ patients on SOWL before and after introduction of DAAs
| Indicator | Measure | Overall 2008–2019 | P1, Years 2008–2013 | P2, Years 2014–2019 | Difference P2 – P1 | Mann-Whitney U test Monte-Carlo-Significance (2-sided) |
|---|---|---|---|---|---|---|
| A) Listed on SOWL by requested organ | ||||||
| Any organ | Sum | 328 | 229 | 99 | –130 | P = 0.004 (99% CI = 0.002 to 0.006) |
| Mean | 27.33 | 38.17 | 16.5 | –21.67 | ||
| SD | 13.51 | 8.21 | 7.23 | |||
| Liver | Sum | 284 | 197 | 87 | –110 | P = 0.004 (99% CI = 0.002 to 0.005) |
| Mean | 23.67 | 32.83 | 14.5 | –18.33 | ||
| SD | 11.56 | 7.65 | 5.82 | |||
| Kidney | Sum | 38 | 28 | 10 | –18 | P = 0.031 (99% CI = 0.027 to 0.036) |
| Mean | 3.17 | 4.67 | 1.67 | –3 | ||
| SD | 2.38 | 1.86 | 1.86 | |||
| B) Delisted from SOWL by reason | ||||||
| Transplanted | Sum | 215 | 141 | 74 | –67 | P = 0.010 (99% CI = 0.007 to 0.012) |
| Mean | 17.92 | 23.5 | 12.33 | –11.17 | ||
| SD | 7.68 | 4.42 | 5.96 | |||
| Not transplanted | Sum | 28 | 21 | 7 | –14 | P = 0.049 (99% CI = 0.043 to 0.054) |
| Mean | 4.67 | 3.5 | 1.17 | –2.33 | ||
| SD | 2.27 | 2.43 | 1.47 | |||
| Deceased | Sum | 42 | 33 | 9 | –24 | P < 0.001 (99% CI = 0 to 0.0005) |
| Mean | 3.5 | 5.5 | 1.5 | –4 | ||
| SD | 2.54 | 1.76 | 1.22 | |||
CI: confidence interval
We found significant reductions (mean difference P2 – P1) in patients listed for requesting any organ (–21.7, P = 0.004), liver (–18.3, P = 0.004), or kidney (–3.0, P = 0.031) in the period after the introduction of DAAs, compared to the period before the release of these medications. In addition, the mean number of transplanted patients (–11.2, P = 0.010), or because of preceding death (–4, P < 0.001) show a significant reduction. The mean number of patients delisted without transplantation also diminished (–2.3, P = 0.049).
In the growth dynamics of the time series for each period (Figure 1), the lines represent the yearly number of cases, mean, and regression fit with 95% CI bonds of the respective curve estimation. In addition, the equation’s parameter estimates, and coefficient of determination are reported.
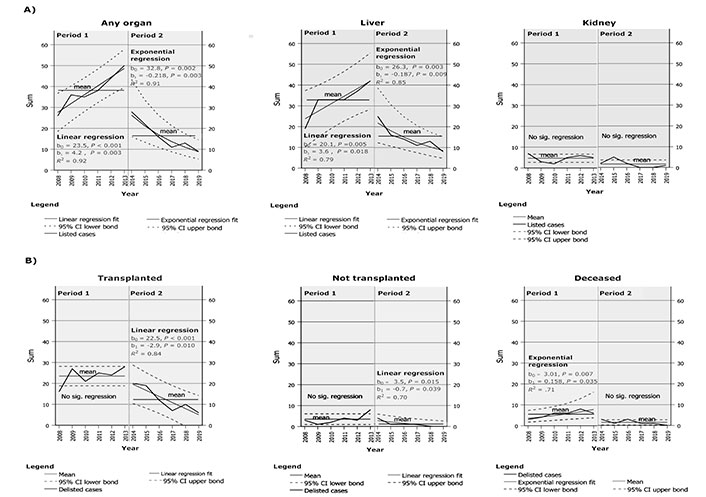
Curve estimations for yearly numbers of HCV RNA+ patients on Swiss transplantation waiting list, comparing periods from 2008 to 2013 with 2014 to 2019. A) Patients listed on, by requested organ. It shows the three panels for reasons of being added to the list (any organ, liver, kidney); B) patients delisted, by reason. It shows three panels for reasons of being removed from the list (transplanted, not transplanted, deceased). For each indicator, a separate panel graphically displays the time series of the yearly listed delisted cases together with their mean, and their growth dynamics as linear or exponential regression estimation fit with 95% CI for period 1 (years 2008–2013, before introduction of DAAs) and period 2 (years 2014–2019, after the introduction of DAAs) as a multiple line graph with the calendar year on the x-axis and the sum of cases on the y-axis. In addition, the calculated parameters of the curve estimation with significance (P-value) and R2 are reported
Visual investigation of the time series showed marked breaks in the development of the yearly numbers of HCV RNA+ patients on the SOWL in 2014, mostly after having peaked in the previous year’s value. From 2013 to 2014 there were conspicuous recesses of patients listed for organ transplantation (any organ: –22%, –44%; liver: –17%, –41%; kidney: –3%, –60%) as well as of the delisted patients (transplanted: –8%, –29%; not transplanted: –4%, –50%, deceased: –3%, –50%).
In addition, we find clear changes in the growth dynamics of the different SOWL indicators, which all occurred from 2013 to 2014, following four different patterns:
The number of patients waiting for any organ, or a liver, showed a trend reversal from a linear increase during the six years before the introduction of DAAs (from 26, respectively, 19 cases in 2008 to 50, respectively, 42 cases in 2013; curve estimations P1: Yt = 23.5 + 4.2 × t, R2 = 0.92, and Yt = 20.1 + 3.6 × t; R2 = 0.79, respectively) to an exponential decrease during the 6 years thereafter [from 28, 25 cases in 2014 to 9, respectively, 8 cases in 2019; curve estimations P2: Yt = 32.8 × e (–0.22 × t), R2 = 0.91, and Yt = 26.3 × e (–0.19 × t); R2 = 0.85, respectively] (pattern 1).
The number of HCV-RNA+ patients waiting for a kidney decreased from a higher stable level in the period before (P1 mean = 4.7; curve estimation P1: no significant regression) to a lower stable level (P2 mean = 1.7; curve estimation P2: no significant regression) thereafter (pattern 2).
The number of delisted patients after transplantation as well as non-transplanted patients showed a change from a stable course during P1 (P1 mean = 23.5, respectively; curve estimations P1: no significant regression) to a linear decrease (from 20, 4 cases in 2014 to 6, 0 cases in 2019, respectively; curve estimations P2: Yt = 22.5 – 2.9 × t; R2 = 0.84, and Yt = 3.5 – 0.7 × t; R2 = 0.70, respectively, pattern 3).
The number of patients delisted due to death grew exponentially during the six years before the introduction of DAAs [from three cases in 2008 to six cases in 2013; curve estimation P1: Yt = 3.0 × e (0.16 × t); R2 = 0.71] and dropped to a stable course thereafter (P2 mean = 1.5; curve estimation P2: no significant regression, pattern 4).
The introduction of HCV DAA in 2014 had a substantial effect on HCV prevalence among individuals on the organ waiting list and on their mortality. The rising trend for organ transplants until 2013 was broken and turned into a declining trend. For obvious reasons, it mainly affects HCV RNA+ people on the liver transplant list. On the renal waiting list, DAAs also significantly reduced the number of HCV RNA+ patients. In parallel, with the introduction of DAA, the mortality of HCV RNA+ individuals on the waiting list decreased.
DAA can be safely used in patients with decompensated cirrhosis with high efficacy [13]. One reason for the decrease in the liver waiting list is that patients who have been successfully treated are being removed. Another reason is the decrease in the number of new cases of HCV-induced decompensated liver cirrhosis and HCC [16]. The prevalence of HCV on the renal waiting list is mainly reduced by the safe use of DAAs even in advanced renal failure [17]. Several studies from Europe and the US have shown comparable results [18–22]. Unfortunately, the welcome effects of DAAs are largely limited to high-income countries [23]. However, the goal of WHO is to eliminate hepatitis C globally. It is not only prohibitive drug prices that represent a substantial hurdle in improving hepatitis C care. Insufficient education, little political awareness, and lack of funds from global donors are also significant reasons why low-income and middle-income countries are unable to successfully combat hepatitis C [23].
However, the positive results of our and other studies do not hide the fact that even wealthy countries are not on track for hepatitis C elimination by 2030 [24]. Of concern is that a significant proportion of patients treated for hepatitis C are still late presenters, which means they have advanced liver disease and/or HCC at treatment initiation [25]. This is entirely considered a systemic failure in rich countries, all of which have broad access to effective hepatitis C testing and treatment. The gaps in the cascade of care in testing and linkage to care need to be addressed rapidly to avoid future organ transplantation in hepatitis C-infected individuals.
A limitation of this study is the absence of access to individual patient data. Therefore, only the information available in the SOWL could be assessed. Due to this, the SOWL could not provide any details regarding potential causes of HCC or reasons for delisting among patients without transplantation. As a result, it was not possible to analyze these factors. In addition, it’s important to note that the results of this study conducted in Switzerland may not be directly comparable to other countries. This is due to a few reasons. Firstly, Switzerland is a relatively small country, which means the small sample size of the study may not be representative of larger countries. Additionally, the availability of drugs for patients with advanced liver disease caused by hepatitis C was unrestricted in Switzerland from the start of approval for DAA treatment, which may not have been the case in other countries that were studied.
In conclusion, DAAs have the potential to substantially reduce the hepatitis C disease burden, as shown using the organ transplant waiting list in Switzerland as an example. To realize this potential, numerous measures are needed in the entire hepatitis C care cascade worldwide, and synergies with other disease control efforts must be exploited.
DAAs: direct-acting antivirals
HCC: hepatocellular carcinoma
HCV: hepatitis C virus
SOWL: Swiss organ transplantation waiting list
SD: standard deviations
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/100532_sup_1.pdf.
LF: Methodology, Formal analysis, Visualization, Validation, Writing—original draft. ST: Investigation, Conceptualization, Data curation, Writing—original draft. PB: Investigation, Supervision, Funding acquisition, Supervision, Resources, Project administration, Validation, Writing—original draft, Writing—review & editing. FI: Conceptualization, Supervision, Resources, Funding acquisition, Project administration, Supervision, Validation, Writing—review & editing.
The authors declare that they have no conflicts of interest.
This retrospective study was performed on anonymous, non-personal data. An assessment by an ethics committee was therefore unnecessary, according to the Swiss ethics regulations.
This study is in accordance with the Swiss federal law on research involving human subjects (Human Research Act, HRA), which allows the working of anonymized aggregated health related personal data without consent to participate.
Not applicable.
Data and materials is available at the corresponding author upon request.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2885
Download: 27
Times Cited: 0
