Affiliation:
1Department of Neuroscience, Center of Biological Sciences, Federal University of Santa Catarina (UFSC), Florianopolis 88040-380, Brazil
Email: kanzlersa@gmail.com
ORCID: https://orcid.org/0000-0002-2394-5002
Affiliation:
2Experimental Neuroscience Laboratory (LaNEx), University of Southern Santa Catarina (UNISUL), Florianopolis 88137-270, Brazil
ORCID: https://orcid.org/0000-0003-4060-5947
Affiliation:
3Department of Gynecology and Obstetrics, Center of Medical Sciences, Federal University of Santa Catarina (UFSC), Florianopolis 88040-380, Brazil
ORCID: https://orcid.org/0000-0003-1978-8443
Affiliation:
1Department of Neuroscience, Center of Biological Sciences, Federal University of Santa Catarina (UFSC), Florianopolis 88040-380, Brazil
4Department of Pharmacology, Center of Biological Sciences, Federal University of Santa Catarina (UFSC), Florianopolis 88040-380, Brazil
ORCID: https://orcid.org/0000-0002-7547-6463
Explor Neuroprot Ther. 2023;3:481–496 DOI: https://doi.org/10.37349/ent.2023.00064
Received: March 21, 2023 Accepted: November 02, 2023 Published: December 26, 2023
Academic Editor: Zixu Mao, Emory University School of Medicine, USA
Aim: To evaluate the application of an acoustic neurostimulation program with binaural beats and isochronic tones isolated or in association, and its effects on sleep, depression, anxiety, and stress in healthy workers.
Methods: A randomized, single-blind, parallel-group clinical trial, using acoustic neurostimulation with binaural beats, isochronic tones, or a combination of these in the 10 Hz range (alpha) performed with daily 20-minute sessions for 21 days. Changes in brainwave patterns were assessed by electroencephalogram (EEG). Psycho-emotional state was assessed with the Depression, Anxiety and Stress Scale 21 Items (DASS-21), and sleep quality with the Pittsburgh Sleep Quality Index (PSQI). In addition, salivary cortisol levels were evaluated as a biomarker of stress.
Results: The data revealed distinct patterns of brainwave modulation via brainwave entrainment (BWE) techniques. Binaural beats and isochronic tones, alone and in combination, effectively increased alpha brainwaves in the temporoparietal region. However, when assessing theta brainwave frequencies in the same region, only binaural beats showed a significant effect. Furthermore, in the prefrontal cortex, an elevation in beta waves was exclusively observed with the use of binaural beats. These findings underscore the specificity of BWE techniques on different brainwave frequencies and regions. The study demonstrated marked improvements in several symptoms related to stress, depression, anxiety, assessed by psychometry with DASS-21 and related to sleep quality assessed by the PSQI.
Conclusions: These results indicate that 10 Hz acoustic neurostimulation in the alpha range, whether through binaural beats, isochronic tones, or a combination of both, can significantly influence brainwave patterns and intensity. Notably, participants exhibited decrease in symptoms of stress, depression, and anxiety, coupled with improved sleep quality. These data suggest that alpha acoustic neurostimulation holds promise as an effective intervention for bolstering mood, mental health, and overall emotional well-being [Brazilian Clinical Trials Registry (ReBec, ensaiosclinicos.gov.br) identifier: RBR-10yj42dj].
Quality of life at work refers not only to adequate working conditions and good socio-professional relationships but also to the fundamental balance of the aspects of human biopsychosocial and organizational needs [1]. According to Kensbock and colleagues [2], we are currently facing an epidemic of mental disorders in the workplace, and the main diseases/disorders related to quality of life in the organizational environment are anxiety crises, panic conditions, depression, stress, sleep disorders, and Burnout syndrome.
To achieve the objectives of this study, an acoustic neurostimulation protocol was used as a non-pharmacological therapeutic approach with daily use, to improve the general quality of life of workers in the corporate environment of Santa Catarina Water and Sanitation Company (CASAN in the city of Florianópolis, Brazil). In auditory perception, when a sound stimulus with known and standardized intensity and frequency occurs in both ears, the reproduction of the same stimulus with small differences in intensity and frequency, the human brain processes an illusory perception of pulses or beats resulting from the auditory information captured through these sound stimuli [3, 4]. This phenomenon was described in 1839 by the German scientist Heinrich Wilhelm Dove, who named them binaural beats (BB). Later, the American biophysicist Gerald Oster described in detail BB parameters and the bases for the perception of these stimuli [5]. Nowadays, it is well recognized that when receiving these stimuli, the brain produces electrochemical discharges that characterize the states and/or levels of consciousness, described in pioneering research using electroencephalogram (EEG) data capture techniques [6].
By using EEG, it is possible to identify the basic features of brain functioning, demonstrated by the patterns of wave frequencies: gamma (30–70 Hz), beta (13–30 Hz), alpha (8–13 Hz), theta (4–8 Hz), and delta (1–4 Hz) [6]. Each of these waves has been correlated with states of human consciousness, and established as patterns such as the awake state, relaxation, sleep and rapid eye movement (REM) phase, among others [7]. Since that, an increased number of studies have described the potential of neural stimulation with BB as a cognitive [8, 9] and neurobehavioral [10–12] enhancer approach. These studies have shown that BB stimulation can modulate cognitive and behavioral processes, suggesting similar effects on the modulation of the brain’s electrical activity patterns [13–15].
Isochronic tones (IT) acoustic neurostimulation is a technique of auditory brainwave entrainment (BWE) also known as neural synchronization. It is a phenomenon in which external stimuli influence the neural oscillations related to specific cognitive states [16], a mechanism in which a single tone is reproduced in regular beat intervals. Although IT are widely used as a form of BWE, there are few studies supporting its effects so far, particularly for cortical alpha entrainment through IT [17].
IT have also the potential to affect neuronal functioning in different ways, thus impacting the individual’s mental state. For instance, these tones consist of short pulses of a single frequency, separated by a fixed fraction of time [17]. The sound pulses are identical in terms of frequency and amplitude for all sound channels through which they are reproduced, thus not requiring a binaural listening condition where exclusively the left channel is delivered to the left ear and vice versa. Consequently, this characteristic makes IT suitable for cases that require a monaural listening of sound, such as individuals with one-ear hearing, monophonic sound reproduction systems, or, in extreme cases, any listening conditions that, as previously mentioned, do not exclusively deliver each channel to each ear. The main characteristics of binaural sounds and IT are presented in Table S1.
In a recent systematic review [18], converging evidence from multiple studies was presented, indicating that BB or isochronic sound stimulations temporarily affect the predominance of a specific frequency of brainwaves, a phenomenon namely brainwave or neural entrainment, that has been associated with benefits on focus, sleep, concentration, and relaxation [19–21].
In this current study, acoustic neurostimulation has been utilized, which has several technical advantages, including the fact that it is very safe and non-invasive, as well as versatile since the therapy can be applied with phone applications or online systems, which allows for remote or home-based stimulation. However, an important limitation of this methodology is its application in individuals with hearing loss, particularly in aged subjects [22]. These auditory stimuli commonly are used to modulate “mental state”, which includes affective balance, internal well-being, preserved consciousness, functional cognitive functions such as attention, memory, and language, as well as motivation for personal goals and objectives [23, 24].
With the advent of EEG, it became possible to study the modulation patterns of brain electrical activity by binaural stimulation (BB) [25, 26] and its effects on psychophysiological, cognitive, emotional, and psychopathological states [26]. For example, Lane et al. [13] described that presenting BB acoustic stimulation of 16 Hz or 24 Hz can increase performance in system tasks, implying the degree of activation or alertness of conscious wakefulness, and, consequently, decision-making capacity [27–30].
Based on previous studies, auditory stimuli through BB or IT have a wide therapeutic range, improving cerebral blood flow, stimulating neuroplasticity, and compensating neurophysiological processes between brain hemispheres [20]. In this context, the present study investigated the impact of acoustic neurostimulation through BB and IT, isolated or in association, and the effects on EEG, quality of sleep, symptoms of stress, anxiety, and depression, as well as salivary cortisol, in healthy workers.
This is a randomized clinical study (RCS) with a group of healthy volunteers without mental illness and/or undergoing psychiatric treatment. The study was registered in the Brazilian Register of Clinical Trials (ReBEC) platform under the number RBR-10yj42dj and is available at the link: https://ensaiosclinicos.gov.br/rg/RBR-10yj42dj.
For the details of the experimental design utilized in the current study, see Figure S1.
The population studied was composed of volunteers and employees of the state company CASAN Florianópolis, Brazil, without mental illness or chemical dependence diagnoses, injuries, or debilitating diseases, and declared healthy. It is known that sample size calculations for non-parametric methods (such as Mann-Whitney, Friedmann, Wilcoxon, and Kruskal-Wallis tests) [31]. The number of individuals surveyed per group was calculated by using the G*Power (Heinrich-Heine-Universität Düsseldorf) software version 3.1.9.4 [32], considering a definition power of 80% and an initial effect of 45%, based on previous studies described in the literature [33]. After the application of the inclusion and exclusion criteria (described below), from the 48 participants recruited by voluntary adherence, 9 individuals were excluded as they did not meet the inclusion criteria, and 2 individuals who contracted COVID-19 dropped off due to quarantine. A total of 37 volunteers remained, with 5 withdrawals for personal reasons that were communicated verbally to the main researcher. Therefore, the sample size of the study consisted of 37 adult volunteers aged between 18 and 70, 35.14% male and 64.86% female, considering a definition power of 90% and an effect of 62% of the study, see Figure S2.
Inclusion criteria: healthy volunteers of both genders, 18 years old or over, with no history of mental illness or substance dependence, and signing the free and informed consent term.
Exclusion criteria: Subjects who did not meet the inclusion criteria, as well as those with psychosis, schizophrenia, epilepsy, or a history of abuse of psychoactive substances, people with hearing problems, or those already using brainwave dragging technology, were excluded from the study.
Participation in the research was voluntary and unpaid, each participant was informed that they could interrupt the experimental session at any time, without the need for technical or medical justifications. The experimental procedure was conducted through a convenience sample. A questionnaire was used to register the participants’ information, such as educational level, age, marital status, and gender (Supplementary material). The details of the participants’ selection are illustrated in Figure S3.
The volunteers were distributed using the software algorithm, without primary selection by gender or age, into four groups of the clinical trial: white noise (control), BB, IT, and the association of BB + IT. The randomization was carried out by a professional not directly involved in the study who was responsible for identifying the electronic files containing the acoustic stimuli according to the stimulation protocol proposed in this study, only with the identification of the individual, without the identification of the experimental group (control, BB, IT, or BB + IT), with monocle masking [34]. The randomization was performed using the Research Randomizer version 4.0 software [35], accessed on December 18, 2021 at http://www.randomizer.org/.
Individuals were randomly assigned their standardized therapeutic protocols, following baseline EEG recordings and psychometric tests, either BB, IT, a mix of BB and IT (BB + IT), or white noise as the control pattern, as follows:
(a) group 0—white noise (white noise-10 Hz), 20 min daily.
(b) group 1—IT + BB (10 Hz) with white noise, 20 min daily.
(c) group 2—BB (10 Hz) with white noise, 20 min daily.
(d) group 3—IT (10 Hz) with white noise, 20 min daily.
Note: standardized and sustained stimulation for a 20-minute therapy with auditory stimuli, with one daily application for 21 days.
Auditory MP3 files with BB, IT, or BB + IT, as well as white noise, were created using the BrainTap Technologies, Inc. software (New Bern, NC, USA). Each stimulus was elaborated separately according to its physical wave nature (white noise, binaural, isochronic, and mix of binaural and isochronic). All sessions started in the beta range (adaptation) and oscillated between 13 Hz and 38 Hz in the first 2 min; then dropped to the alpha range (entrainment), oscillating from 8 Hz to 12 Hz from 3 min to 18 min; then it returned to the beta range, oscillating from 13 Hz to 38 Hz, from 18 min to 20 min (recognition).
Data were collected by means of neurometric evaluations (EEG), six times in two distinct events (1 and 2), the initial and acute treatment, and after 21 days of treatment, the final event configuring the neuroacoustic applications in the volunteers, as shown in the Table S2.
Psychometric analyses for depression, anxiety, and stress [the Depression, Anxiety and Stress Scale 21 Items (DASS-21)] and the Pittsburgh Sleep Quality Index (PSQI), as well as biochemical analysis of free salivary cortisol levels, were collected twice: at T0 (baseline) and T1 (post-treatment). Additionally, heart rate (HR), and systolic and diastolic arterial pressure [pulmonary arterial systolic (PAS)] measurements were also evaluated before and after the acoustic neurostimulation. In summary, the assessments were conducted:
(a) Changes in EEG oscillations, in the stimulated groups, and in the control group, were recorded at the following time points:
event 1: pre-treatment, treatment, and post-treatment;
event 2: pre-treatment, treatment, and post-treatment.
(b) Psychometry data related to depression, anxiety, and stress symptoms, between T0 and T1, addressed by using DASS-21.
(c) Changes in the sleep pattern during the treatment process compared to the patient’s historical record between T0 and T1, were evaluated in the PSQI instrument.
(d) Changes in the concentration of free salivary cortisol, between T0 and T1.
The individuals were instructed to report the use of medications and drugs that may alter brain functions, such as antidepressants, stimulants (caffeine, nicotine, and others), and anticonvulsants; not to undergo the EEG recording in a fasted state; and not to perform physical activities at least 1 h before the test. The volunteers were subjected to blood pressure and HR checks for EEG triage. Electrode area points (forehead and posterior part of the ears) were cleansed with 70% alcohol, with cotton soaked for 30 s, The points were allowed to dry for 30 s to avoid any interference of the alcohol in the application of the electrodes. EEG data were recorded using the MUSETM device (InteraXon Inc., Toronto, ON, Canada).
The MUSE headset EEG uses four recording channels (TP9, TP10, AF7, and AF8), and a fifth reference channel located in Fpz (10–20 international system) for capture and research. The equipment performs robust digital signal processing on-board, with noise filtering and the use of fast Fourier transform (FFT) in real time [36] using the 10–20 international system. The raw EEG brain signals were captured with the Mind Monitor system algorithm version 2.3.1 (James Clutterbuck), accessed on May 13, 2021, at https://mind-monitor.com/. Each signal capture channel on the scalp of the skull (TP9, TP10, AF7, and AF8) registers measurements in microvolts (mV). The raw EEG values were processed using the FFT to generate the absolute numerical values. FFT was used to calculate the power spectral density of each frequency in each channel. Basically, it shows which frequencies compose a signal and “how much” of each frequency is present. Each channel contains 129 decimal values with a range of approximately –40.0 to 20.0. Each drag represents the FFT coefficients (expressed as power spectral density) for each channel, within a frequency range of 0–110 Hz, divided into 129 compartments. The system uses a Hamming window of 256 samples (at 220 Hz), where then, for the next FFT, a window of 22 samples (1/10 of a second) is displaced. These values were emitted at 10 Hz.
An adapted version of DASS-21 for Brazilian Portuguese was used in this research, which contains the three subscales for assessing symptoms of depression, anxiety, and stress [36]. On the DASS-21, participants indicated the degree to which they experienced each of the symptoms described in the items during the past week on a 4-point Likert scale from 0 (does not apply to me) to 3 (applies to me a lot, or most of the time). Scores for symptoms of depression, anxiety, and stress are determined by the sum of the scores of the 21 items [37].
PSQI is a standardized, simple, and well-accepted questionnaire developed by Buysse et al. [38] to assess sleep quality and disturbances during a specific and quantified period of a directed study. The instrument consists of 19 self-report questions categorized into seven components, graded from 0 (no difficulty) to 3 (severe difficulty).
A total score greater than 5 indicates that the individual is experiencing major dysfunctions in at least two components, or moderate dysfunction in at least three components.
Saliva samples were obtained by a non-invasive procedure [tube with Salivette® swab, order number: 51.1534.500 (SARSTEDT AG & Co. KG, Germany)] and were collected at the CASAN outpatient clinic between 9 in the morning and 2 in the afternoon. This technique may be used to study the circadian rhythm of cortisol and to evaluate the hypothalamus-hypophysis-adrenal (HHA) axis in situations of stress, anxiety, and depression, and in the evaluation of sleep deprivation. Changes in salivary cortisol concentration were measured by chemiluminescence in the intervals between T0 and T1.
After reading and signing the free and informed consent form, the volunteers answered the identification questionnaire and the psychometric tests DASS-21 and PSQI. Participants were then submitted to the EEG baseline assessment followed by a 20-minute acoustic neurostimulation session according to group assignment (BB, IT, or BB + IT). EEG was also recorded during and immediately after the session. Subsequently, the volunteers underwent one daily remote acoustic neurostimulation session for 21 days. The sessions in MP3 format are played with the help of a cellphone or computer application. After the last home session, the volunteers were asked to complete the DASS-21 and the PSQI and submit them to an acoustic neurostimulation session. Blood pressure (PAS) and HR was assessed before and after neuroacoustic sessions and EEG sampling was performed before, during and after acoustic neurostimulation. A summarized scheme of the experimental design and utilized procedures is presented in Table S3.
Data distribution and normality were assessed with the Kolmogorov-Smirnov test (KS). Sphericity was not met so data were analyzed by the non-parametric Friedman and Wilcoxon test. A significance level of 0.10 (10%) was used in the analysis. A slightly higher statistical error than usually used (5%) was chosen due to the low sampling. The confidence interval was 90%. The statistical analyses were performed using the SPSS version 20 (IBM, Armonk, NY) and Minitab version 16 (Minitab, LLC., State College, PA) software.
The statistical analyses indicated changes in wave patterns and the presence of BWE phenomenon, detected in the EEG in the alpha frequency range in BB (P-value = 0.075) and BB + IT groups (P-value = 0.098), shown in Figure 1A, delta waves by BB (P-value = 0.046) in Figure 1B, theta waves in Figure 1C, by BB (P-value = 0.021), all in the temporoparietal region and beta waves by IT (P-value = 0.031) in the frontal region (Figure 1D).
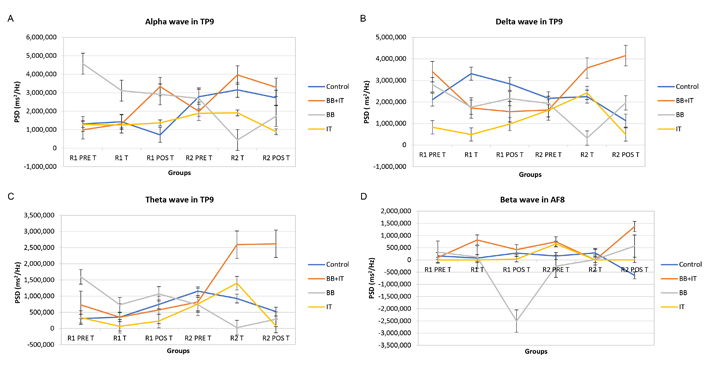
Effects of acoustic neurostimulation with BB and IT alone or associated (BB + IT) on electrophysiological responses evaluated in the EEG. PDS of alpha (A), delta (B), theta (C), and beta (D) waves. Statistical significance level: P ≤ 0.100 (*), 0.01 < P < 0.05 (**), P < 0.01 (***). PDS: power spectral density; R1 PRE T: round 1, pre-workout (application); R1 T: round 1, training; R1 POS T: round 1, post-workout; R2 PRE T: round 2, pre-workout; R2 T: round 2, training; R2 POS T: round 2, post-workout
Conversely, when analyzing the data in the gamma wave patterns, given the extremely high dispersion of data from all wave patterns and acoustic stimuli, there were no statistically significant differences (Figure 2).
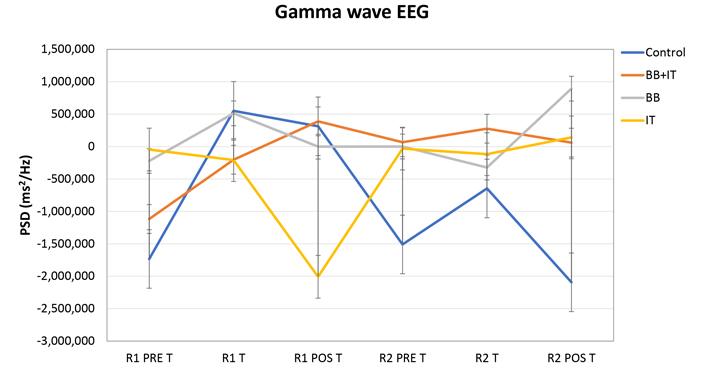
Effects of acoustic neurostimulation with BB and IT alone or associated (BB + IT) on electrophysiological responses evaluated in the EEG. PDS of gamma waves. Statistical significance level: P ≤ 0.10 (*), 0.01 < P < 0.05 (**), P < 0.01 (***)
In the first round of treatment (T0), the symptoms of depression, anxiety, and stress of the participants measured using the DASS-21 (total) scale revealed a mean sample score of 14.43 and in the second round (T1) the score was 6.54. In the result of DASS-total score, statistical significance in the DASS-total score was found in the BB and IT groups. In BB, the average fell from 21.78 in T0 to 7.67 in T1 (P-value = 0.008) and in the IT group (P-value = 0.028) the average also fell from 15.29 to 4.00 (Figure 3A).
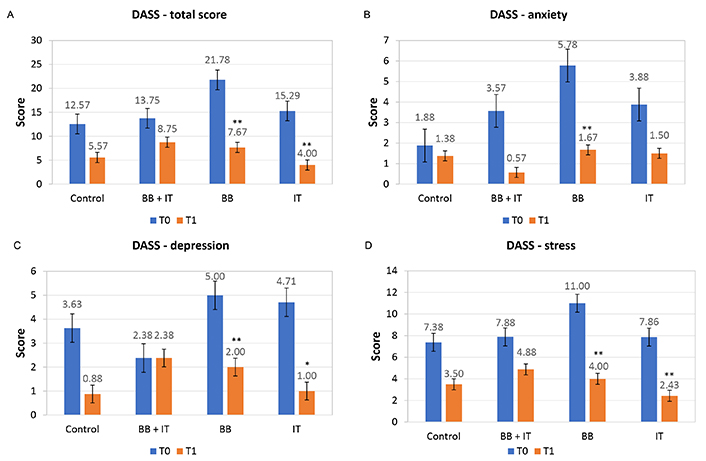
Effects of acoustic neurostimulation with BB and IT alone or associated (BB + IT) on total score (A) and symptoms of anxiety (B), depression (C), and stress (D) evaluated by DASS-21 test. Statistical significance level: P ≤ 0.10 (*), 0.01 < P < 0.05 (**), P < 0.01 (***)
For the anxiety symptoms subscale, 22 subjects (59.45%) reported normal scores (score: 0–6); 5 subjects (13.51%) reported mild anxiety symptoms (score: 7–9); 3 subjects (8.1%) reported moderate anxiety (score: 10–14); and 7 subjects (18.92%) reported severe and extremely severe anxiety symptoms (score: 15–42). In the second round of treatment, after 21 days, the scores at T1, 3 subjects (9.38%) reported mild anxiety (score: 7–9) and another 29 individuals (90.62%) had a normal score (score: 0–6). After statistical treatment of the data with the Friedmann non-parametric test, the group treated with BB (P-value = 0.028) showed statistical relevance, with the means in T0 of 5.78 falling to 1.67 in T1, while the association of BB + IT and IT alone did not show statistical significance (Figure 3B).
For the subscale of depression symptoms, 24 subjects (64.86%) were considered to have a normal score (score: 0–9); 7 individuals (18.94%), reported mild depressive symptoms (score: 10–12); 3 subjects (8.10%) presented moderate depressive symptoms (score: 13–20); and 3 subjects (8.10%), reported severe to extremely severe depressive symptoms (score: 21–42). In the second round of treatment after 21 days, the scores in T1 found that only 1 subject presented symptom compatible with moderate degree 3.10% (score: 13–20), while 96.90% of those subjects tested presented a normal score (score: 0–9). Statistically, the results for DASS-depression were significantly altered by BB stimuli (P-value = 0.035) with the means in T0 of 5 falling to 2 in T1. With IT stimulus (P-value = 0.068), the means were 7.86 in T0 falling to 2.43 in T1, while the association of BB + IT did not present statistical significance (Figure 3C).
For the stress subscale, 19 subjects (51.38%) reported normal scores (score: < 10); 3 subjects (8.1%) reported mild signs of stress (score: 11–18); 6 subjects (16.2%) reported moderate stress (score: 19–26); and 9 subjects (24.32%) reported signs of severe to extremely severe stress (score: 27–42). On the second round of treatment (T1), after 21 days, 1 subject (3.12%) reported mild symptoms of stress (score: 11–18), 4 individuals (12.50%) reported signs of moderate stress (score: 19–26), and 27 subjects (84.38%) had a normal score (score: < 10). After statistical treatment of the data with the Friedmann non-parametric test, the group treated with BB (P-value = 0.012) showed statistical relevance, with the mean in T0 of 11 falling to 4 in T1, while the group stimulated by IT (P-value = 0.027) the means in T0 were 7.86, falling to 2.43 in T1, while the association of BB + IT did not show statistical significance (Figure 3D).
When comparing the results obtained between T0 (pre-treatment) data and T1 (post-treatment of 21 days), a significant reduction in the total score of the global sleep quality evaluation, in the order of 26.90%, was based on the scores and means with a standard deviation of the sleep components that constitute the PSQI instrument, as illustrated in Figure 4A (PSQI global). We highlight the groups stimulated with BB + IT (P-value = 0.075), with the mean at T0 of 7.38 falling to 4.25 at T1 and IT (P-value = 0.041), with the means at T0 of 4.71 falling to 2.71 at T1, which individually pointed out statistically significant differences between pre- and post-treatment, while the BB group showed no changes between T0 and T1 (Figure 4).
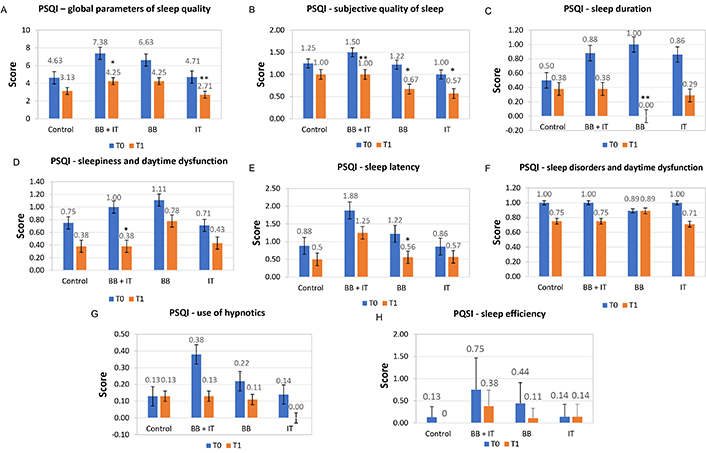
Effects of acoustic neurostimulation with BB and IT alone or associated (BB + IT) on global parameters of sleep quality (A), subjective quality of sleep (B), sleep duration (C), sleepiness and daytime dysfunction (D), sleep latency (E), sleep disorders and daytime dysfunction (F), use of hypnotics (G), and sleep efficiency (H) using the PSQI. Statistical significance: P ≤ 0.10 (*), 0.01 < P < 0.05 (**), P < 0.01 (***)
All groups presented significant alterations in the self-referenced subjective quality of sleep component, where BB + IT (P-value = 0.046), with means in T0 of 1.50 falling to 1 in T1, while the BB group (P-value = 0.059), with means in T0 of 1.22 falling to 0.67 in T1, and the IT-stimulated group (P-value = 0.083), with means in T0 of 1.00 falling to 0.57 in T1 (Figure 4B). When analyzing the sleep duration component, the BB-stimulated group (P-value = 0.041), with means in T0 of 1 fell to 0 in T1 (Figure 4C). In the sleepiness and daytime dysfunction component, the BB + IT-stimulated group (P-value = 0.059), with means in T0 of 1.00 falling to 0.38 in T1 (Figure 4D). In the sleep latency component, once again the BB-stimulated group (P-value = 0.084), with means in T0 of 1.22 falling to 0.56 in T1 (Figure 4E). The other sleep quality components of the PSQI (sleep disorders, use of hypnotics, and sleep efficiency) did not show significant changes when comparing pre- and post-treatment scores (Figure 4F, G, and H).
No significant differences were observed between the groups in free salivary cortisol when comparing pre- and post-treatment (T0 and T1). However, it should be noted that the group treated with IT presented P-values (0.121) close to the acceptance limit, showing a statistical tendency, with the means at T0 being 0.226 and at T1 falling to 0.129 in ng/dL (Figure 5).
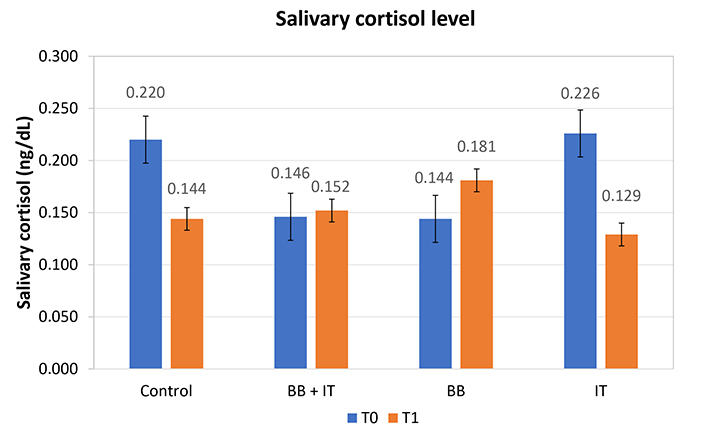
Effects of acoustic neurostimulation with BB and IT alone or associated (BB + IT) on salivary cortisol levels. Statistical significance level: P ≤ 0.10 (*), 0.01 < P < 0.05 (**), P < 0.01 (***)
EEG analysis provided insight into the role of neuromodulation, BWE, and inter-hemispheric cerebral coherence. The evidence points to the presence of the BWE phenomenon, detected in EEG in the alpha frequency band by BB (entrainment, and inter-hemispheric brain coherence). Results demonstrated that the auditory stimulation protocol used induced significant changes in EEG after the 21-day treatment period (stimulation by BB, IT, or BB + IT).
The current results corroborate previous findings from the literature [39, 40]. However, the difference in prolonged and daily exposure for 20 min with BB and BB + IT over 21 days may represent the determining factor for the predominance of the alpha brainwave in the groups stimulated by BB and BB + IT. On the other hand, these results suggest that brain entrainment (BWE) requires continuous and frequent stimuli, a factor that can cause neuroadaptations in pathways involved in responses related to stress, depression, anxiety, and sleep quality. Considering that the predominance of alpha waves produces relaxation, a possible role of this wave pattern in reducing the levels of stress, anxiety, and depression observed in this study and measured by the DASS-21 instrument, may be considered.
Previous evidence suggests a functional subdivision into two classes of the alpha band [40–42]. The first subdivision, alpha 1, or low alpha, covers the frequency range between 8 Hz and 10 Hz, located prominently in the occipital lobe in low-resolution electromagnetic tomography (LORETA) images. The alpha 2 subdivision comprises the frequency range between 10 Hz and 12 Hz or 13 Hz, detected in the precuneus region and in areas of the medial occipital parietal cortex [43]. It has been associated with the state of vigilance and alertness not connected to a specific thought or stimulus [43].
Regarding the theta rhythm, during wakefulness, there are two patterns of neural activity of this rhythm that reflect distinct states of attention [39, 44, 45]. Still related to the frequency pattern of theta waves (4 Hz to 8 Hz), considering the evidence that these waves are related to sources of neural activity in the anterior cingulate gyrus with projections to the superior region of the frontal cortex [42, 46], with a high prevalence during the initial stages of sleep [47, 48]. The first pattern is represented by a diffuse distribution and is linked to a decrease in the body’s level of alertness, associated with drowsiness [45, 49]. The second pattern of theta activity, attributed to the frontal midline pattern, positively correlates with performance on tasks that require focused attention, mental effort, and more efficient stimulus processing [43–45]. In the present study, a predominance of theta waves was observed in the temporoparietal region, suggesting their role in reducing the level of alertness, possibly regulated by the broad cortical activation of the ascending reticular activating system (ARAS). In this case, it interferes with the quality and efficiency of sleep. Furthermore, several brain regions of the limbic system, mainly sept hippocampal connections, show a predominance of theta neural activity [42]. In the medial frontal region, theta rhythm neural activity has been associated with the monitoring of response outcome behavior, functions in processes related to long-term memory, cognitive performance, spatial exploration, and REM sleep.
Classically, it is recognized that the theta rhythm originates from neuronal activations in the brainstem, including noradrenergic neurons of the locus coeruleus and serotonergic neurons of the dorsal raphe nucleus. Stimulation of these cells, whether through collateral sensory input or through cortical feedback pathways, causes stimulation of the septal nuclei, the brain area where the propagation of theta oscillations begins [43, 44]. This stimulation also facilitates the flow of transmission and processing of information between different structures of the limbic system [45].
Using Bayesian Belief Network analysis (BBN, 4–7 Hz for 20 min) in young adults post-physical training, a marked increase in parasympathetic activation and self-reported relaxation was observed [42]. In another study with a single intervention with IT at 6, 10, and 40 Hz for 5 min, it reduced anxiety symptoms and improved reports of well-being in healthy individuals [22]. Comparing our findings with these previous results, it can conclude that the current protocol (10 Hz) produced effects on the parameters of stress, anxiety, and depression. This suggests that the frequency and conditioning of the stimulus influence the flow generation of the theta wave pattern and its increase in activity in the various cortical structures related to the limbic system and emotional regulation.
In our experiment with acoustic neurostimulation (10 Hz), delta wave patterns were identified in the EEG in the temporoparietal region when stimulated with BB. The delta rhythm (0.5–4 Hz) is particularly associated with neural activities in healthy adults during sleep and is involved in potentially inhibitory neural activities [43]. The presence of delta-type oscillations distributed in the temporoparietal region may be associated with the quality of non-REM sleep, specifically, delta wave sleep, known as phase N3 [50]. Therefore, the finding indicated that stimulation with BB may have facilitated the induction of deep sleep in the volunteers in the present study. Regarding the increase in beta waves in the frontal region detected in our study when stimulated by IT, it must be considered that, in general, the origin of oscillations in the beta frequency range is related to cortico-cortical connections [48]. This band was also subdivided into two frequency bands. The first, beta 1, is defined as the frequency range between 13 Hz and 21 Hz and can be located bilaterally in the frontal cortex, mainly reflecting the local activation state of the cortex [45]. The beta 2 band (21.0 Hz to 25.5 Hz) has been associated with higher concentration requirements. Therefore, in the case of increased cognitive demand, both subdivisions of the beta frequency band tend to increase their magnitude in the EEG spectrum.
When there is dominance of neural activity in the beta band of the EEG spectrum, recorded at rest, this pattern has been associated with symptoms of generalized anxiety, irritability, agitation, and sleep disorders [51]. Such data implies that IT stimulation at a frequency of 10 Hz and regulation of the beta band may induce anxiolytic effects, improving sleep quality. Finally, the results showed that gamma-band oscillations showed no evidence of statistical significance in our EEG study.
The results observed in the present study regarding the parameters evaluated in the total DASS-21 psychometric instrument show that stimulation with BB and IT alone was effective in relation to the stress, anxiety, and depression triad or their interconnection. However, the two associated stimuli (BB + IT) did not induce the same response.
These results suggest that the type of acoustic stimulus should be adjusted for related therapeutic targets, as the DASS-21 scale is based on the tripartite model in which symptoms of anxiety and depression are grouped. They are defined by the presence of negative affect, depressed mood, insomnia, discomfort, and irritability. There are also specific symptoms of depression such as anhedonia, absence of positive affect, and specific symptoms of anxiety such as somatic tension and hyperactivity [36, 37], which constitute a wide range of symptoms.
Regarding the assessment of sleep quality using the PSQI instrument, a general improvement in sleep was observed with BB + IT and IT stimulation alone. There was also an improvement in sleep duration and latency with BB. In terms of subjective sleep quality, the groups stimulated with BB, BB + IT, and IT showed statistically significant differences. With the associated stimulation of BB + IT, there was an improvement in daytime sleepiness in the studied groups, modulating these sleep components. These are essential factors to contribute to sleep hygiene and improve individuals’ quality of life. These results may be related to the change in the subject’s brainwave pattern (alpha and theta) when an input from the alpha wave generator (10 Hz) is induced in the central nervous system (CNS). This change may alter the neuroendocrine regulation related to the circadian cycle, a finding that demands new studies on this parameter related to sleep quality. The sleep complaints most frequently observed in most emotional or mental disorders are related to the difficulty in initiating and maintaining sleep (latency and maintenance of sleep duration, respectively), sleep efficiency, and disturbed sleep. Early awakening is more related to depressive disorders. In association with this, the percentage of REM sleep is increased in emotional disorders, and the reduction in latency for REM sleep is mainly described for depression but can appear in other pathologies [49]. Even more relevant is the close relationship between inadequate sleep hygiene and various psycho-emotional patterns, which worsen patients’ symptoms and quality of life [50–52].
Each type of brainwave may modulate different neurotransmitter systems, inducing specific synaptic and neurochemical adjustments [43, 44]. In this research, observations point to the effectiveness of BB in neuromodulation, which involves mitigating emotional states of anxiety, stress, and depression, contributing to neuroadaptation and brain homeostasis. On the other hand, it was also evident that IT can influence behaviors associated with stress and depression, reducing the symptoms of these conditions, according to the self-perception of the interviewees. Therefore, we can suggest that both stimuli are effective but act through different mechanisms of neuroplastic neuromodulation of neuronal pathways and regulation of neurotransmitters in different ways, corroborating previous data described in the literature [26].
Our results support the view of the therapeutic potential of acoustic neurostimulation. For more rational and appropriate clinical use, it is necessary to refine clinical protocols. This refinement requires a deeper understanding of these phenomena to elucidate and evaluate the methods’ effectiveness, concerning their influence on brainwave patterns and the consequent modulation of mood and mental health.
This research provides original evidence of the benefits of acoustic stimuli on mental health based on regulating sleep and reducing symptoms of stress, anxiety, and depression. Additionally, we can suggest that BWE through acoustic neurostimulation using BB or IT, although still little explored in behavioral and neurophysiological therapy, may represent an effective, inexpensive therapeutic approach with minimal side effects.
It is expected that this study will stimulate the interest of the scientific community in the development of research with acoustic stimuli and other forms of neuromodulation. This interest opens new perspectives in non-pharmacological therapies for the maintenance and prevention of mental health and potential therapy for emotional disorders.
The present results indicate that 10 Hz (alpha) acoustic neurostimulation with BB, IT, or BB + IT can modulate the intensity of neuronal oscillations and brainwave patterns. It can also modulate stress, depression, anxiety, and sleep quality. These data suggest the hypothesis that alpha acoustic neurostimulation may prove useful in improving mood, mental health, and emotional well-being. Further, larger-sized studies are necessary to validate this hypothesis.
The present study was carried out during the COVID-19 pandemic, which negatively impacted the number of volunteers and imposed obstacles due to the social isolation the global scenario required. However, even with such limitations, the study was conducted safely, adopting all the measures recommended by the World Health Organization.
Another limitation is that there are only a few rigorous clinical studies on this issue. Therefore, this essay aims to contribute to the literature, provide greater support to health professionals, and consolidate the safe use of this therapeutic modality. The effects presented in the research open up a significant possibility of therapeutic use but also require further studies for safety in its multiprofessional application. Finally, the data needs adaptation for the transposition to daily treatment of patients with mental disorders, especially concerning the training of qualified professionals to apply the therapy.
BB: binaural beats
BWE: brainwave entrainment
CASAN: Santa Catarina Water and Sanitation Company
DASS-21: Depression, Anxiety and Stress Scale 21 Items
EEG: electroencephalogram
FFT: fast Fourier transform
HR: heart rate
IT: isochronic tones
PSQI: Pittsburgh Sleep Quality Index
REM: rapid eye movement
The supplementary Figures and Tables for this article are available at: https://www.explorationpub.com/uploads/Article/file/100464_sup_1.pdf. The supplementary questionnaire for this article is available at: https://www.explorationpub.com/uploads/Article/file/100464_sup_2.pdf.
We would like to thank Companhia Catarinense de Águas e Saneamento of the state of Santa Catarina (CASAN), for providing the physical space and access to the volunteer employees who participated in this research and BrainTap Technologies, Inc., USA, for the cooperation, construction and provision of standardized MP3 audios, used for acoustic neurostimulation in the tested groups.
SAK: Conceptualization, Investigation, Writing—original draft. FJCF: Conceptualization, Writing—original draft. BK: Investigation. RDP: Writing—original draft. All authors have read and agreed to the published version of the manuscript.
The authors declare no conflict of interest.
The study was submitted to the Ethics Committee for Research Involving Human Beings at Ethics Committee for Research with Human Beings-Federal University of Santa Catarina (UFSC-CEPSH) and approved under number 5,102,377 on November 12, 2021.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The datasets analyzed for this study can be found at https://repositorio.ufsc.br/bitstream/handle/123456789/249853/PGNC0370-T.pdf?sequence=1&isAllowed=y.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
