Affiliation:
1Pacific Institute for Research and Evaluation, Beltsville, MD 20705, USA
Email: fisher@pire.org
ORCID: https://orcid.org/0000-0002-3823-3438
Affiliation:
1Pacific Institute for Research and Evaluation, Beltsville, MD 20705, USA
ORCID: https://orcid.org/0000-0001-9537-2066
Affiliation:
1Pacific Institute for Research and Evaluation, Beltsville, MD 20705, USA
ORCID: https://orcid.org/0000-0003-1099-8743
Affiliation:
2Charles Stewart Mott School of Public Health, College of Human Medicine, Michigan State University, Flint, MI 48502, USA
ORCID: https://orcid.org/0000-0002-0958-2639
Explor Digit Health Technol. 2025;3:101171 DOI: https://doi.org/10.37349/edht.2025.101171
Received: April 08, 2025 Accepted: September 23, 2025 Published: November 09, 2025
Academic Editor: Subho Chakrabarti, Postgraduate Institute of Medical Education and Research (PGIMER), India
The article belongs to the special issue Telepsychiatry in Low-and Middle-income Countries: an Update
We describe the rationale for and design of a non-inferiority trial to evaluate the relative effectiveness of electronic alcohol screening with in-person vs. electronic brief intervention (BI) approaches implemented in Alexandra Township, South Africa, and Zacatecas-Guadalupe, Mexico. The purpose of screening and brief intervention is to identify individuals whose responses to the Alcohol Use Disorders Identification Test (AUDIT) indicate risky drinking patterns and offer them information and advice to help them reduce their drinking. We seek to determine whether a BI comprising information and advice delivered electronically, along with the opportunity to schedule an appointment with a health care professional at a later time, is not significantly worse than a more labor-intensive traditional BI provided through a face-to-face interaction with a health professional immediately following screening. Selected patients visiting participating health clinics in Alexandra and Zacatecas-Guadalupe will be asked to complete the AUDIT screening using an online app accessed via a handheld device. Those whose scores indicate risky alcohol consumption will be invited to participate in the study. Participants at the clinics will be allocated in alternate weeks to either a customary in-person BI or an electronic BI. Based on power analyses taking attrition and nesting within clinics into account, the target sample sizes are 680 in Alexandra and 560 in Zacatecas-Guadalupe. Measures of 30-day alcohol consumption and AUDIT scores will be obtained at baseline, 3 months, and 6 months. The primary outcome will be the past 30-day quantity-frequency of alcohol consumption. Outcomes will be compared for the two study conditions using mixed effects multilevel regression analyses to account for nesting of observations within participants and participants within clinics. Potential socio-demographic covariates include gender, age, marital status, the highest completed level of education, family’s primary native language (a proxy for ethnicity/culture), presence of household members younger than 16, and subjective economic status (Trial ID: NCT07150156. Clinical trial platform: ClinicalTrials.gov Protocol Registration and Results System. Web address: https://clinicaltrials.gov).
Alcohol use and misuse remain a primary health concern worldwide. In 2019, 2.6 million deaths were directly attributable to alcohol consumption. Of the world’s population aged at least 15, 7.0% had an alcohol use disorder (AUD) and 3.7% were dependent on alcohol. Alcohol use has been linked to over 200 adverse health conditions, such as liver and heart disease, cancer, and a variety of adverse mental health and behavioral conditions. Worldwide in 2019, 4.7% of all deaths were attributed to alcohol, with the highest levels of alcohol-attributable deaths in the World Health Organization (WHO) African and European regions. Alcohol consumption is also associated with harm to others, including fetal alcohol spectrum disorders, crime and violence, and deaths attributable to alcohol-related road crashes [1, 2]. Although most of these alcohol-related harms derive from heavy episodic or continuous use of the substance, it is increasingly recognized that no level of use is risk-free [3, 4].
Screening and brief intervention (SBI) constitutes an effective strategy—endorsed by the WHO—that is designed to reduce hazardous and harmful drinking patterns and their associated negative consequences [5, 6]. Despite evidence of its effectiveness, access to SBI remains limited [7], particularly in low- and middle-income countries (LMICs) that have a relatively high alcohol-attributable disease burden and health systems that are often overwhelmed and unable to provide specialized services for AUDs [8, 9]. Given that much of the projected increase in alcohol consumption and harmful use of alcohol is expected to occur in LMICs, scalable strategies are urgently needed to address these alcohol-related health disparities, including the potential to employ non-specialist health workers and lay counselors to deliver interventions for a range of disorders, including AUDs [10].
Traditionally, the initial screening component of SBI has been conducted by health care providers (HCPs) such as doctors or nurses in a face-to-face interaction with their patients as a component of their regularly scheduled medical care. The screening component is often administered by using the Alcohol Use Disorders Identification Test (AUDIT), a brief 10-item instrument [6, 11, 12]. Typically, patients who screen positive for harmful or hazardous drinking are then given a brief intervention (BI) that may include a motivational interview by clinic staff to help patients reduce or discontinue their risky alcohol consumption [13]. BIs provide patients with general alcohol health information about the risks associated with alcohol consumption and the benefits of reducing or quitting drinking. Motivational interviews may also probe for acceptance of personal responsibility for risky drinking and readiness to change and engage patients in goal setting, which includes inviting patients to make a realistic and personally tailored plan to change their patterns of alcohol consumption, assisting them with managing social contexts that present an elevated risk for consumption, and referring them to treatment if their AUDIT scores are sufficiently elevated [14].
Studies of the barriers to implementation of SBI have shown that health professionals in a variety of countries perceive that it is costly and time-consuming, of uncertain efficacy, and challenging to administer successfully [10, 15]. Another concern is that patients are likely to deny the extent of their alcohol use and its potential consequences, and resist advice concerning reduction [16]. Further, access to SBI by populations worldwide is low [17], especially among those living in LMICs, where uptake by health clinic staff is constrained by time limitations and competing responsibilities [10]. Less labor-intensive—and thus more economical—alternatives to traditional SBI administration could increase SBI reach and penetration. In addition to using trained non-medical personnel, digital SBI applications delivered via personal computers, telephones, or handheld devices constitute another promising alternative. Although current electronic SBI (eSBI) typically lacks the ability to deliver a personalized, therapeutic interaction like that provided by a clinician, it can administer and score the AUDIT and provide tailored feedback concerning the level of risk associated with a patient’s drinking patterns. Moreover, because digital technology is developing rapidly, it can provide normative feedback concerning patients’ consumption patterns relative to those of others and assess their readiness to change their behaviors to which BIs could then be tailored [18].
To reduce hazardous and harmful drinking by promoting greater accessibility to SBI, the AB InBev Foundation (ABIF) funded the development of an eSBI tool that has been available online to the general public. This tool, in the form of a web-based app, automates the administration and scoring of the AUDIT and delivers an electronic brief intervention (eBI) to those whose AUDIT scores total at least 8. That eBI includes an explanation of the screening results and provides access to videos tailored to different risk levels and a brochure with information on the health benefits of reducing alcohol consumption as well as advice on strategies for attaining that goal. The eBI also facilitates patients scheduling a visit with an HCP or other professional to answer their questions and, for those whose AUDIT scores are 15 or higher, to obtain counseling to help address their risky drinking or alcohol dependence. ABIF contracted with the nonprofit Pacific Institute for Research and Evaluation (PIRE) to conduct an independent non-inferiority evaluation to determine whether eBI produces significantly worse outcomes than the more labor-intensive traditional in-person BI. Because the screening component of the intervention will be administered electronically via the app for all participants, the primary research question PIRE will address is whether the electronically delivered BI is significantly less effective than the BI that is conducted entirely face-to-face with clinic staff.
Previous trials of eSBI have yielded mixed results, in part due to variations in the populations represented in the studies and differences in the nature of the non-eSBI interventions used as comparators. In one trial involving university students in which eSBI was examined relative to an informational pamphlet only, it was associated with reductions in alcohol use, heavy episodic drinking, and consumption-related social problems. Of these, only the latter was significant 6 months post-intervention [19]. In a second study, students reporting unhealthy levels of alcohol use were randomly assigned either to a control group that received an informational pamphlet only or to a single or multiple session eSBI group. At 9-month follow-up, participants in both of the intervention groups reported fewer academic problems [20]. In a third study, students reporting an elevated risk of hazardous drinking were randomly assigned to an electronic screening condition only or to eSBI. At 6-month follow-up, those receiving eSBI reported larger reductions in drinking frequency, volume of alcohol consumed, academic problems, and alcohol-related chronic problems than those receiving the screening condition only [21]. Another study that delivered SBI to adolescents by computer compared to delivery by nurse practitioners found no differences between the conditions in any of the alcohol-related behaviors studied, including use, binge drinking, and sex while intoxicated [22].
An Australian study of adult drinkers randomly assigned people with hazardous or harmful drinking either to electronic screening or screening plus additional assessment and personalized feedback all delivered electronically (eSBI) [23]. The personalized feedback included: participants’ scores on additional assessment measures and an explanation of their meaning, an estimated blood alcohol concentration for the heaviest drinking episode with information on relative traffic crash risk, an estimate of their yearly expenditures on alcohol, and bar graphs comparing their consumption levels to medical recommendations and those of adults of the same age and gender. The personalized feedback also offered information about alcohol, tips for reducing the risk of harm, and sources of support for drinking problems. At 6- and 12-month follow-ups, no significant differences existed between the two groups on any of the six primary and secondary drinking outcomes.
A recent meta-analysis reported findings from 201 clinical trials involving 94,753 participants comprising non-treatment-seeking harmful and hazardous drinkers in online, health, or community settings. These trials, which did not include a no-treatment group, compared practitioner to electronic delivery of interventions to reduce alcohol consumption and heavy drinking episodes. Early (i.e., 1- and 6-month) positive differences in alcohol consumption were noted for the practitioner condition, but these differences were attenuated in follow-up 1 year later. Heavy episodic drinking did not differ between the two conditions [24]. An earlier meta-analysis, which focused on the effects of digital technologies as applied to youth, reviewed three evaluations of the relative effects of eSBI on alcohol use and reported that any early effects favoring either in-person or eSBI dissipated quickly [25–27]. Another meta-analysis compared the effects of eSBI relative to the regular standard of care as reported by four randomized controlled trials comprising 2,641 alcohol-related trauma patients. eSBI reduced problematic alcohol consumption up to 6 months following implementation [28].
Findings from a meta-analytic review of evaluations of electronic screening, brief intervention, and referral to treatment (eSBIRT), which includes referral to treatment for screened individuals whose scores indicate they are at high risk for substance use disorders and may need specialty care, concluded that the evidentiary base supporting the intervention was weak. Although it reduced the frequency of alcohol consumption, any effects were only temporary. The authors also noted that variability in the design and implementation of eSBIRT interventions across disparate studies was a complicating factor in efforts to aggregate and interpret their findings [29]. Of particular relevance to the current investigation, a systematic review of 19 studies conducted in LMICs reported that digital interventions that were partly human-delivered were more effective than those that were exclusively delivered digitally [30].
This evaluation is designed as a non-inferiority trial. Unlike traditional trials, in which the evaluator seeks to determine how likely a novel intervention is to achieve the objectives of a program under study relative to a no-treatment control group, a non-inferiority trial seeks to establish whether the effects of a novel intervention are at least equivalent to those of the comparison condition [31]. Thus, our central research question is: Are BIs that are primarily administered by an electronic app with the option of a later in-person meeting no less effective than those that are administered entirely face-to-face by clinic staff?
At the start of the study, one site will be assigned to conduct traditional BI in the first week and the other to start with eBI in the first week. For each site, all eligible patients will be provided with the designated intervention throughout the week. The following week, clinics at each site will offer the other condition. The conditions then will be offered in alternate weeks. Based on power analyses, data collection will continue until at least 680 participants have been recruited in Alexandra and 560 in Zacatecas-Guadelupe, approximately half in each BI condition at each site.
One setting for this non-inferiority trial comprises health clinics in Alexandra, a primarily poor, Black, and densely populated township with an estimated 180,000 people in Johannesburg, South Africa. Alexandra has a high crime rate and low access to basic services [32]. Its two predominant languages are Zulu and Northern Sotho [33].
The seven participating local health clinics all provide primary care. Some also specialize in specific chronic conditions (e.g., HIV, diabetes, or behavioral health). Trained facilitators at each participating clinic will interact with the patients waiting to receive medical care to implement the screenings, collect study data, and for in-person BI weeks, conduct a face-to-face intervention.
The other trial setting is the metropolitan area, including Zacatecas and its sister municipality, Guadalupe, Mexico. Zacatecas is the capital and largest city of the state of Zacatecas in north-central Mexico. As a World Heritage Site, a major part of Zacatecas’ economy comes from hosting festivals and tourism along with silver mining, commerce, and manufacturing, notably beer production. In 2020, the Zacatecas-Guadalupe metropolitan population was 405,000 [34]. The predominant language in Zacatecas and Guadalupe is Spanish.
Nine health clinics providing primary care in Zacatecas-Guadalupe will participate in the trial. The health clinics are part of the IMSS Bienestar public health services system that serves Mexicans not covered by the private health system or the system for state workers. Trained facilitators at the participating clinics will interact with adults in clinic waiting rooms, both patients waiting for appointments or those accompanying patients, to implement the screenings and the BIs (i.e., digital and face-to-face in alternating weeks) as in South Africa and collect study data.
Patients aged 18 or older waiting for appointments will be approached by the trained facilitator and asked to complete the alcohol screening on an electronic handheld device. Because any drinking during pregnancy carries risks, especially to the fetus, all women who are approached to take the screening will first be asked by the facilitator if they are or may be pregnant or if they are breastfeeding; those who are will be excluded from the trial. They will be administered the screening by the facilitator and then given a face-to-face BI that includes a strong abstinence message.
With support from ABIF, the health clinics participating in this study are providing SBI to their patients via the eSBI app, which can be used to provide both components electronically or to administer the screening electronically and then an HCP can take over and administer a traditional face-to-face BI. During the course of this trial, the clinics in South Africa will integrate the app into the clinic’s routine patient registration and check-in process. In Mexico, IMSS Bienestar has given permission for the study to be conducted in nine of its clinics, but the app is not being integrated into the clinic processes of the health system and will not feed into the patients’ electronic health records. All participating clinics at the two sites will use the app to administer the AUDIT to as many patients as possible using a clinic-provided electronic device. Because the non-inferiority trial is being grafted onto a subset of the overall number of SBIs the clinics in both sites are conducting, a version of the app has been designed for the study that provides the trial’s informed consent script and survey to people drinking in a high-risk pattern immediately following the AUDIT and before the BI is delivered. This study version of the web-based app will be used until the clinics in each site obtain at least the targeted number of completed surveys, approximately half of them in the eBI and half in the traditional BI conditions.
In South Africa, all components on the eSBI app for the study (AUDIT screening, consent script, study survey, BI) are available in English, Zulu, and Sotho; in Mexico, all components are presented in Spanish. Patients who are comfortable using the electronic device and able to read the materials will self-administer the AUDIT before proceeding to other components for which they may qualify, including the consent script and the survey. Those who are not able to read or are not comfortable using the device may request that the facilitator assist them by reading aloud the questions and recording their responses. Based on information provided by our in-country collaborators/contractors, we anticipated that about 40% of patients in South Africa would ask the facilitator to administer the data collection orally; in Mexico, this percentage was estimated to be less than 15%. Facilitators in both sites have extensive experience administering the screening and take appropriate steps to ensure privacy and confidentiality by moving with the patient to an area in the waiting room away from others or to another room to prevent anyone from hearing the survey questions and the patient’s responses.
The AUDIT comprises 10 questions, the first three of which constitute the AUDIT-C, or short form [6]. Patients who score at least 4 on the AUDIT-C will then be administered the remaining seven AUDIT items. Adults at least 18 years of age with total scores of 8 or more on the full 10-item AUDIT—indicating hazardous or harmful drinking—will qualify for the study.
The eSBI app will invite patients with qualifying AUDIT scores to answer two eligibility items about alcohol screenings at the clinic within the past 6 months so that those who were potentially exposed to SBI recently can be excluded from the study. Those eligible for the study will then be presented with the study informed consent script and, if they agree to participate, will be asked to complete the baseline survey on the app. Only patients who indicate their willingness to participate in the study by clicking the agree button will be presented with the survey.
As in the screening, participants will either read and self-administer the survey questions or they may ask the facilitator to read the questions aloud to them and record their answers. Participants screened in weeks when the BI will be delivered electronically will be sent to the results screen to receive their eBI after completing the study survey and the tracking information section designed to collect contact information for conducting outreach for the follow-up surveys. Those screened in traditional BI weeks will return the device to the facilitator after completing the survey and tracking information and then receive a face-to-face BI. Participants in the baseline survey will be recontacted to complete follow-up surveys at 3 months and 6 months; however, there will be no BI delivered as part of the follow-up surveys. Figure 1 displays a flow diagram of the SBI and the study recruitment process.
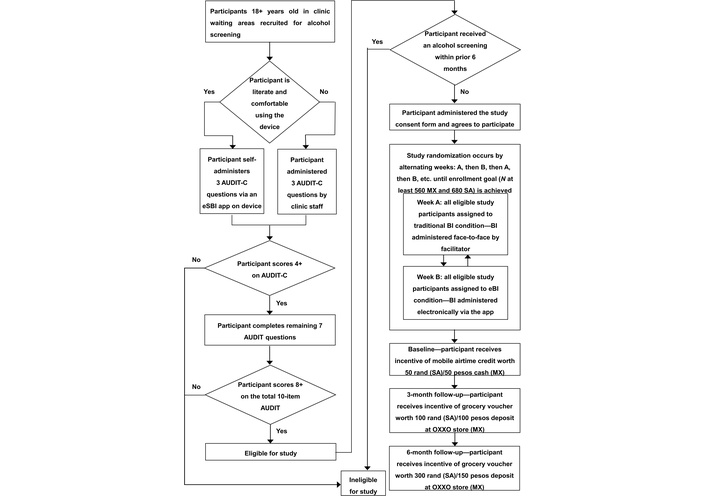
e-SBI non-inferiority trial: participant flow. AUDIT: Alcohol Use Disorders Identification Test; BI: brief intervention; eBI: electronic brief intervention; eSBI: electronic screening and brief intervention; MX: Mexico; SA: South Africa.
Participants in both sites will receive incentives of increasing value over time to compensate them for their time spent completing the survey and for remaining in the study. In South Africa, participants in the baseline survey will receive a mobile airtime credit of 50 rand (≈ 2.70 USD). Respondents in the 3-month and 6-month follow-up surveys will receive grocery vouchers worth 100 rand (≈ 5.41 USD) and 300 rand (≈ 16.22 USD), respectively. In Mexico, the incentives will be 50 pesos (≈ 2.45 USD) in cash for the baseline survey and deposits in the participants’ names that can be redeemed for cash at the extensive network of OXXO convenience stores and gas stations for 100 pesos (≈ 4.90 USD) and 150 pesos (≈ 7.35 USD) for the 3- and 6-month surveys, respectively.
Participants in the eBI condition will receive information about their AUDIT score in the form of a results screen that will interpret their score by displaying one of five screens tailored to the severity of their alcohol use. A figure with an arrowhead indicates the level of risk associated with a participant’s score along a continuum ranging from “no risk” (for abstainers) to “low risk” (AUDIT scores 1–7) to “moderate risk” (AUDIT scores 8–14) to “high risk” (AUDIT scores 15–19) and, lastly, to “alcohol dependency” (AUDIT scores 20 and higher). To decrease the risk of negative consequences resulting from their alcohol consumption, participants who score 8 or higher on the AUDIT will be advised to reduce how much they consume and drink on fewer occasions. They will also be encouraged to schedule an appointment with an HCP who will address any of their questions. At this point, they will be invited to sign up for a personal and confidential account they can use to request an appointment with an HCP and download their screening answers for later review or to share with an HCP. Those who request an appointment with an HCP via the app in South Africa will see a clinic social worker for counseling and assistance, sometimes at a home visit, and, if more intensive treatment is needed, they will be directed to the treatment and rehabilitation clinic. In Mexico, initial referrals will be made to one of the local community centers for mental health and addictions that are part of the IMSS Bienestar health system, which provides evaluation and diagnostic services and can refer those who need more intensive treatment to a residential treatment clinic within the same health system. Those in the two highest risk categories will be warned that their consumption level dangerously increases the risks to their health and safety and advised to immediately seek help from an HCP or, for those in the highest risk category, a provider who can treat alcohol dependence.
In addition, participants in the eBI condition will be given the opportunity at their discretion to click on relevant buttons to access other educational resources, including a brochure tailored to each site and a brief video featuring an authoritative speaker in a lab coat delivering feedback. These materials contain information about the physical and social harms associated with alcohol misuse, the benefits of and strategies for reducing their alcohol consumption, and tips for responsible drinking and for protecting their health and safety when drinking. The brochures also present information on standard drink sizes, safe drinking guidelines for males and females (e.g., maximum drinks per week), and situations in which any alcohol use should be avoided. Although the specific content differs between the sites, conditions under which no alcohol should be consumed include the following: being younger than the minimum legal drinking age; pregnant or breastfeeding; advised by an HCP or pharmacist to abstain from drinking alcohol; in recovery or unable to control the amount of alcohol consumed; have medical conditions or are taking medications for which drinking alcohol is contraindicated; have had a serious mental disorder; have had a history of dependence on alcohol or other substances; and are (or will be) driving, operating machinery, or performing a task for which skill and attention are required to ensure safety. The brochures also remind readers that no level of alcohol consumption is entirely safe. In Mexico, a hard copy of the brochure is available to participants in the eBI condition who request it.
During traditional BI weeks, the facilitator will interact with participants to deliver information and advice tailored to their total AUDIT score and answers to individual screening items, which the facilitator will be able to access on the device. The facilitators are trained to provide alcohol information, brief advice, and, as warranted, referrals. They are trained to probe for additional information about alcohol use and medical conditions; raise awareness of the health and social consequences of the participant’s current drinking, especially in relation to any health issues they are experiencing; discuss benefits of reducing consumption; encourage people to take responsibility for changing their drinking; identify challenges and potential solutions; and assist people in making plans to reduce their alcohol consumption. In South Africa, referrals are first made to a clinic social worker for an appointment, who then can make subsequent referrals as needed to the treatment and rehabilitation center [32]. In Mexico, after the initial referral to a local community clinic for mental health and addictions, patients who need more intensive services may be referred to the residential treatment center. Although the alcohol brochures are one of the resources in the app for those in the eBI condition, in Mexico, traditional BI previously included providing the brochure in hard copy to patients; this will continue in this trial.
Approximately 10 weeks following the beginning of baseline data collection, each site’s study coordinator will query the app’s dashboard to determine which study participants will be due for the 3-month follow-up survey in the next 3 weeks.
The study coordinator will check to see if patients due soon for the 3-month survey have a clinic appointment already scheduled that coincides with the timeframe (i.e., within 3 weeks of the target date) for their follow-up survey. Because Alexandra’s participating health clinics serve patients with chronic conditions, many study participants will return at regular intervals (e.g., monthly, every three months) for medical care. Those who return to the clinic for an appointment within 3 weeks of their follow-up target dates will be asked to complete the 3-month surveys at that time. Those who do not return to the clinic at regular intervals that coincide with the time specified for the follow-up surveys, or who miss an appointment, will be contacted by clinic staff to arrange for a meeting with a clinic social worker at a mutually agreeable time and location to take the survey or offered to take it as a phone interview. Participants who have moved out of the area and who therefore cannot meet in person will also be offered the option of a phone interview. This process will continue each week until all baseline participants have been recontacted for the 3-month survey. These procedures will be repeated at the time of the 6-month follow-up survey.
Because the health clinics in Mexico are primary care clinics that do not have significant populations with serious chronic conditions, most patients will not be returning at regular intervals. After each week’s cohort of baseline participants who are due for their 3-month follow-up has been identified, clinic staff will conduct direct outreach using the participant’s preferred contact method. Prior experience in Zacatecas-Guadalupe suggests that 90% of participants will prefer to be contacted by phone and 10% will prefer to be messaged via text, WhatsApp, or email. Participants will be offered two options for taking the survey: a phone interview or an online survey, most likely completed on their phone. Prior experience suggests that most of those contacted by phone will answer the questions via a short phone interview at the time of the outreach contact. As in South Africa, this process will continue until all baseline participants have been contacted and will be repeated as the time for the 6-month survey approaches.
Study participants in both the traditional BI and eBI groups will be administered the AUDIT via an app presented on a handheld device, which will then invite those who are eligible by virtue of their elevated scores to participate in the study by completing a brief survey. Traditional BIs and eBIs will be implemented in alternating weeks. Those visiting the clinics on eBI weeks will receive feedback through the app that is tailored to their level of risk based on their AUDIT score and will be given the opportunity to watch a video and view a brochure. They will also be given the opportunity to make an appointment for another time to meet with a clinic social worker (South Africa) or to talk to an HCP at the local community mental health and addictions clinic (Mexico). Participants in the traditional BI condition will receive their BI administered face-to-face by the facilitator. Those whose AUDIT scores suggest additional assistance may be beneficial will be referred to a clinic social worker/community clinic. Participants will be recontacted to complete follow-up surveys at 3 months and 6 months.
We will invite study participants to complete three surveys, the first of which will be administered at baseline before they receive any intervention, followed by surveys 3 and 6 months later.
Participants will answer five questions about past 30-day alcohol use (typical frequency, typical quantity, greatest number of drinks in a day, frequency of consuming the greatest number of drinks, and frequency of heavy episodic drinking [i.e., 6+ drinks on one occasion]) as specified in Table 1. These items are widely used in the alcohol research field [35] and are a subset of the questions used in evaluating other initiatives in the ABIF’s Global Smart Drinking Goals Program [36]. Participants will also be asked to provide socio-demographic information about themselves, including their age, gender (male, female, other), marital status, highest completed level of education, family’s primary native language (as a proxy for ethnicity/culture), presence of children younger than 16 in the household, and subjective (i.e., perceived) economic status, as assessed by income and property relative to that of other families in Alexandra or Zacatecas-Guadalupe. Additionally, the clinics will provide us with responses to the 10 AUDIT screening items.
Past 30-day alcohol use items.
| Directions and drink definitions | |
|---|---|
The next questions ask about your use of alcohol in the past 30 days. If you’re unsure about any answer, please make your best guess. South Africa standard drink definitions When we ask about alcoholic drinks, one whole drink is:
Mexico standard drink definitions When we ask about alcoholic drinks, one whole drink is:
| |
| Alcohol use items | Response |
| Alc 1. In the past 30 days, on how many days did you have at least one whole drink, more than a sip or taste, of any alcoholic beverage? | _____ days (0–30) |
| Alc 2. In the past 30 days, on the days when you drank alcohol, about how many whole drinks did you typically have? | _____ drinks (0–100) |
| Alc 3. You said you usually had [number from Alc 2] whole drinks when you drank alcohol. Thinking about the past 30 days, what was the greatest number of drinks with alcohol you had on any one day? | _____ drinks (0–100) |
| Alc 4. In the past 30 days, on how many days did you have [number from Alc 3] alcoholic drinks? | _____ days (0–30) |
| Alc 5. On how many days in the past 30 days did you have 6 or more alcoholic drinks on one occasion? | _____ days (0–30) |
After completing the survey questions, participants will be asked for contact information to assist clinic staff in getting in touch with them for the two follow-up surveys. The facilitator will then record the mode of administration for the survey (i.e., by the patient, by facilitator/clinic staff, or other, such as a friend or family member) so that we can assess mode effects. Two additional questions will ask the facilitators to record whether the participant completed the survey and, if they did not, the reason for the break-off (i.e., their appointment started, they stopped participating, or other). These items will allow us to distinguish participants who dropped out from those who simply exercised their option to skip survey questions that they did not want to answer. Following completion of the tracking information, the facilitators in South Africa will confirm with participants which phone number the mobile airtime credit should be applied to. In Mexico, the facilitator will provide the cash incentive.
For the 3-month follow-up survey, respondents will be administered a subset of items they completed at their baseline clinic visit: the full AUDIT, a smaller number of demographic items, and the five past 30-day alcohol use items. For the 6-month follow-up survey, they will be presented with all the 3-month survey items and, in addition, a satisfaction and behavior change strategies measure designed to assess their perceptions of the SBI they received and any changes they made in the last 6 months to reduce their drinking. The first section asks participants to answer questions about the BI they received at baseline; it invites them to rate on 4-point scales: their ease of understanding and the usefulness of the feedback they received about their alcohol use (screening results), information on the health effects of alcohol misuse, and strategies for reducing drinking. Additional questions ask participants to rate their confidence and perceived self-efficacy to reduce their drinking as a result of engaging in the SBI process (i.e., answering the screening questions and receiving advice). Finally, participants will be asked to report on whether they engaged (Y/N) in a series of strategies in the past 6 months to reduce their drinking, including drinking on fewer days, choosing lower-strength drinks, alternating between alcoholic and non-alcoholic beverages, avoiding situations where people drink, and quitting drinking altogether.
Although the screening is standardized, differences in the way the BIs are implemented between the two conditions may affect the extent and types of information and guidance participants receive. Process data will help identify these disparities in intervention intensity. Across both sites, we will determine whether participants in the eBI condition clicked on the video, accessed the brochure offered on the app, or requested an appointment with an HCP. For those in the traditional BI group, facilitators will document which components they delivered, i.e., alcohol information, brief advice, and referral. After receiving their BIs via the app, study participants in the eBI condition in South Africa may use the app to schedule a meeting with the clinic’s social worker to get questions answered or receive assistance. Those in South Africa in the traditional BI condition may be referred by the facilitator to a social worker for more intensive counseling based on their AUDIT scores. Social workers to whom referrals are made will record whether the participant made an appointment and whether the participant kept it. As part of its ongoing SBI effort, the Foundation provides funding that ensures social workers promptly handle these referrals. However, in Mexico, the initial referrals are not made to clinic staff but to community clinics, which will preclude the collection of follow-up information on the referral process at this site.
Table 2 displays primary and secondary outcomes for this study and their sources.
Primary and secondary drinking outcomes and associated measures.
| Outcome | Measures | Source or questions |
|---|---|---|
| Primary | Past 30-day typical quantity-frequency | Alc 1Alc 2 |
| Secondary | Past 30-day maximum quantity-frequency | Alc 3Alc 4 |
| Past 30-day frequency of 6+ drinks | Alc 5 | |
| Past 3 months 10-item AUDIT score | AUDIT screener |
AUDIT: Alcohol Use Disorders Identification Test.
The analysis strategy for this non-inferiority trial will assess differences in change over time in the outcome measures between study participants who are in the eBI vs. traditional BI condition regardless of what BI components were delivered (i.e., an intent-to-treat analysis). Separate analyses will be conducted using the South Africa and Mexico samples followed by analyses of the combined data from the two sites. The primary outcome of interest (Table 2) is the past 30-day quantity-frequency of drinking transformed into grams of alcohol consumed. Supplemental analyses will explore the secondary outcomes (30-day maximum quantity-frequency, 30-day frequency of 6+ drinks, and AUDIT score).
Analyses for the separate samples will comprise multilevel mixed effects regression models that account for nesting of observations within participants and participants within clinics to evaluate changes in the outcomes from baseline through the 3- and 6-month follow-ups. Linear and Poisson models will be used for continuous (e.g., quantity-frequency) and count (e.g., frequency of 6+ drinks) variables, respectively. Zero-inflated or negative exponential models will be used where appropriate. Participants’ study condition (eBI vs. traditional BI) will be coded as a dummy variable for these analyses and will represent the exposure variable. Survey mode (self-administered, read to the participant, or telephone) will be coded as two dummy variables for each wave of data collection and included as a covariate in all models. Time (baseline, 3-month follow-up, 6-month follow-up) will be treated as an ordinal variable. The previously described socio-demographic variables will be included as covariates in these analyses. To help account for factors that may influence the characteristics of participants recruited into the sample given the eBI and face-to-face BI are administered in alternating weeks, an ordinal variable representing consecutive two-week blocks of time during the intervention period will be created and included in the main analyses if significant associations with baseline alcohol consumption are observed. The primary effect of interest in these analyses is the time × study condition interaction to assess whether observed changes in the outcomes for the eBI group differ from those for the traditional BI group. In addition to the intent-to-treat analyses, we will explore whether the intensity of the delivered intervention moderates the intervention effects on the outcomes. The intensity measures include whether participants in the eBI condition scheduled an appointment with an HCP and accessed the app-based brochure and video, and the number of elements (i.e., alcohol information, brief advice, referral) that were completed in the delivery of traditional BIs. In addition, sensitivity analyses will be used to explore if survey mode or scheduling and attending an in-person consultation with a counselor moderated intervention effects.
Analyses of the combined data will parallel those for the separate samples, but will include three levels with observations nested within participants, participants within clinics, and clinics within sites. The primary effects of interest are the study condition × time and site × time × study condition interactions to explore whether there is overall evidence for the non-inferiority of the eBI intervention and for differences in the relative effects of eBI across the two sites.
As secondary analyses, satisfaction with eBI versus traditional BI will be explored through cross-sectional multilevel linear regression models. These analyses will address both the overall mean satisfaction across all the items and for the individual items. Logistic regression analyses will be conducted for the self-reports of behavior change strategies, and depending on the dispersion statistic, Poisson or negative binomial regressions for overall counts across the items will be used to examine differences across the two BI conditions. All models will include the condition indicator and the background covariates as predictors.
Although our analytic approach can accommodate participants with differing numbers of data points, we will conduct attrition analyses (χ2 and logistic regression analyses) at each wave to investigate how those remaining in the study differ from those who dropped out, including whether there are differences in attrition between the eBI and traditional BI conditions and between the sites. If systematic differences are found, we will model attrition using a probit analysis and include a selection variable (inverse Mills ratio) in the primary analyses to help correct for selection bias [37]. We will similarly model participation rates and whether they differ across sites and study conditions (eBI vs. traditional BI) and will include a selection variable in the analyses if necessary.
We anticipate low rates of item missingness because the surveys are administered on devices or by a trained facilitator who reads the items to a participant and records the responses. Nonetheless, we will probe for systematic patterns of missingness at the item level using logistic regression analyses (e.g., missingness associated with treatment condition, age, sex). In addition to complete case analyses of the outcomes, we will employ an expectation-maximization (EM) approach to impute missing items if the data are missing completely at random (MCAR) based on Little’s test [38] or appear likely to be missing at random (MAR) based on the missing data analyses. Sensitivity analyses will be conducted to assess the robustness of our results and conclusions based on each approach.
Following guidelines for non-inferiority trials [39], the power analysis was based on the effect of the eBI being at least 50% of the expected lower bound for the effect of the face-to-face BI to which we are comparing it. A recent systematic review of the alcohol SBI literature estimated that this lower bound is –28 g of alcohol per week [40, 41]. Thus, to be considered non-inferior, the eBI would need to produce a mean reduction in drinking of at least 14 g of alcohol per week (δ = 0.35). Assuming modest nesting within clinics (rho = 0.01) and a 25% attrition rate, the target sample to attain 80% power for the expected effect size was 680 for Alexandra with 7 clinics and 560 for Zacatecas-Guadalupe with 9 clinics. For the analyses of the combined samples with 1,240 participants nested in 16 clinics within two sites, power is 80% for a smaller effect size (δ = 0.25).
In this non-inferiority trial, we contrast the effects of two modalities of delivering a BI to clinic patients residing in a low-income township in South Africa and in a secondary city in Mexico and whose scores on an alcohol use screening tool indicate the need to reduce their risk of harmful consequences. We expect to find no differences between the two conditions; that is, that eBI is not significantly worse than a more labor-intensive traditional in-person BI in reducing alcohol use in our targeted high-risk population. If the less costly and less labor-intensive eBI is found to be non-inferior to the traditional BI, it would have important implications for the implementation of alcohol interventions in LMICs where resources may be limited.
This clinical trial comes with some limitations. First, because this is a non-inferiority study, we will not enroll a true control group that does not receive any of the components of a BI. Rather, participants receiving the eBI will be compared with those receiving a face-to-face BI, which is the treatment as usual already being delivered in the participating clinics. As a result, we will not know if any observed changes in drinking differ from those that would have been observed if no intervention had been implemented. However, a literature exists showing that SBI is an effective intervention for reducing drinking [40, 41], which supports the use of a non-inferiority trial. Moreover, the choice to use a non-inferiority trial is based both on its acceptability to our local partners and the ethical considerations that would arise from denying needed services to patients at the clinics. Nonetheless, any positive changes following the in-person BI or eBI could possibly be attributed to historical effects of which we are unaware (e.g., changes in service hours or practices in local drinking establishments; crackdowns on illegal or quasi-legal establishments; local economic downturns that reduce disposable income). Any such effects, however, would be consistent across the eBI and traditional BI conditions and thus should not bias the non-inferiority comparisons, although they limit our ability to draw conclusions about the absolute size of the intervention effects on alcohol consumption. Similarly, because the interventions were implemented in alternate weeks, it is possible that events during a given week might affect who is recruited into the interventions. However, these events are not expected to be systematically associated with the study conditions. Nonetheless, we include an indicator of when during the intervention phase each participant was exposed to the eBI or in-person BI to help account for any such effects.
Second, neither the administration of SBI in the eBI condition nor in the traditional BI comparison group constitutes a “pure” type of service delivery. For example, SBI has traditionally relied on an HCP to deliver and score the AUDIT; in this study, the screening task in both conditions will be conducted electronically via the app, consistently and, we believe, without scoring errors. That said, we expect that as many as 40% of the enrolled participants in South Africa and 15% in Mexico will elect to have the AUDIT read aloud to them because they are insufficiently literate to read and understand the questions or because they are uncomfortable using the electronic device. Although we will be able to identify those who ask for assistance and will include this as a covariate in our analyses, we expect that participants may be more reticent to admit to heavy drinking when the survey is not self-administered. An indicator of survey administration mode at each data collection wave will be included as a covariate in the analyses. Additionally, there are aspects of the eBI delivery that overlap with the traditional BI, including the opportunity to make an appointment with an HCP. This option was included in the public version of the app for use by the general public taking the SBI remotely, which was developed prior to this study. After taking their screening and receiving their feedback through the app, the public version lets respondents search for HCPs near them and make an appointment. This capability was maintained when the study version of the app was developed. So, while the eBI can be considered a primarily although not purely digital mode of delivery, we imagine that ultimately research may find that the most successful mode of SBI will be a hybrid that offers information provided by respondents and feedback delivered to them in a digital format, with elements of traditional BI integrated such as the ability to have a discussion with an HCP.
Relatedly, because participants in the traditional BI group are expected to have immediate access to a face-to-face BI delivered by the facilitator, they may benefit more from the program than participants in the eBI group. For the eBI group, the intervention is more likely to be limited to the information shared via the app, unless they elect to set up (and then keep) a later appointment with an HCP. Participants’ exposure to information and guidance about reducing alcohol consumption is largely under the control of the facilitator in traditional BIs, whereas it depends to a much greater extent on participants’ motivation and proactivity to access and process the feedback in eBIs. That distinction is critical to understanding potential differences in the depth of content delivered by the two modes. Another difference is that eBIs cannot probe for new information from participants or personalize the feedback to the same extent that in-person BIs offer. These differences in the depth of content and personalization are a function of the service delivery mode and the constraints in current eBI applications. For these reasons, it is important to assess the outcomes of the two delivery modes. To account for differences in content depth as much as possible in our analyses, we have included measures of the intensity of the BI.
Another limitation is that the follow-up period is relatively short. Many longitudinal studies [42], but by no means all [43, 44], include a measurement a year or more following the intervention. Although a longer-term follow-up is desirable, we believe a 6-month follow-up is adequate to establish non-inferiority for at least moderate-term gains. Future randomized controlled trials should address the effectiveness of eBI with longer follow-ups.
Finally, study participants receive modest incentives that increase over time to compensate them for remaining engaged in the study. As a result, participation rates may not mirror those that would be observed in other settings where incentives are not provided or are less salient. In addition, given the incentives, there may be some selection biases; those participating in the study may not reflect the entire population of risky drinking patients in the clinics. However, the incentives are modest and provided only for participation in the research surveys, not for engaging in the interventions. Moreover, potential participants are not informed about the incentives until after they have completed the screening and qualified for the study, making it highly unlikely that they could affect responses to the AUDIT screener. In addition, any selection or response biases resulting from the incentives would be consistent for both study conditions and thus should not bias the comparisons of the eBI and traditional BI groups.
Ultimately, we believe that this study’s findings are likely to be suggestive, not conclusive: The current eSBI is not driven by artificial intelligence, so the question of the relative effectiveness of traditional versus fully electronic delivery of SBI will remain an open one. In that regard, the quality of the eBI mode of delivery should increase substantially over time, as improvements in artificial intelligence enhance the realism of the dialogue between human and machine by supporting a robust virtual discussion between the two. That means future iterations of the app should become ever more sophisticated and effective insofar as they include personalized feedback about the patient’s drinking and its health consequences, and fully operationalize the principles and practices of motivational interviewing. The potential for eSBI to be administered outside of a clinical context—like any other interactive app, and in conditions of privacy that do not require revealing to an HCP behavior that may be considered shameful or embarrassing—is also promising.
In conclusion, we note that the findings from the study described here can be generalized to the field of substance abuse treatment only with considerable caution, in that the characteristics of the SBIs offered in the trial sites in Alexandra Township, South Africa, and Zacatecas-Guadalupe, Mexico, may not be representative of those offered in other geographic and cultural contexts. It is likely, for example, that motivational interviews offered elsewhere will differ in duration and content, particularly as to the inclusion of personalized feedback, goal setting, and action plan development. It is also likely to differ by the training, competence, sincerity, autonomy, and status of those delivering the service [29, 45]. In addition, BIs are likely to differ across sites and cultures as to the degree to which the encounter between the HCP and patient is primarily one of information giving—that is, unidirectional—or whether the provider engages the patient in an interactive discussion characterized by mutual respect and inquiry.
ABIF: AB InBev Foundation
AUD: alcohol use disorder
AUDIT: Alcohol Use Disorders Identification Test
BI: brief intervention
eBI: electronic brief intervention
eSBI: electronic screening and brief intervention
eSBIRT: electronic screening, brief intervention, and referral to treatment
HCPs: health care providers
LMICs: low- and middle-income countries
PIRE: Pacific Institute for Research and Evaluation
SBI: screening and brief intervention
WHO: World Health Organization
The content is solely the responsibility of the authors and does not necessarily represent the views of the AB InBev Foundation, AB InBev, or any of their affiliates.
The authors gratefully acknowledge Tom Achoki and Angela Rizzo, who are staff of our sponsor; Gustavo Ramos Hernández, Lulama Sulupha, and Virginia Malete, who are local collaborators at the sites and are responsible for delivering the intervention and collecting the data; Elena Cárdenas Vargas and Josephine Tshabalala, who serve as local collaborators with the Mexico and South Africa sites, respectively, and are paid consultants of ABIF, for assisting us in supervising the local program delivery and data collection effort.
DAF: Conceptualization, Methodology, Project administration, Resources, Visualization, Writing—original draft, Writing—review & editing. CLR: Conceptualization, Methodology, Visualization, Writing—original draft, Writing—review & editing. JWG: Conceptualization, Methodology, Resources, Writing—original draft, Writing—review & editing. TRM: Conceptualization, Funding acquisition, Methodology, Supervision, Writing—review & editing. All authors read and approved the submitted version.
The authors have been supported for the past 8 years by funding from the AB InBev Foundation to independently evaluate its programs to reduce harmful alcohol use. The sponsor, whose staff are acknowledged, did not review this manuscript prior to its publication.
PIRE’s institutional review board (IRB) (FWA00003078) reviewed and approved the research protocols used in both South Africa and Mexico. Our collaborators in Mexico submitted the procedures to be used there to a local research ethics committee, Comité de Ética en Investigación Hospital General Zacatecas “Luz González Cosio” (CONBIOÉTICA-32-CEI-001-20231023), which granted approval for the study. Supplemental local approval was not required in South Africa. As required by US regulations, the project’s human subjects protections are compliant with the ethical principles and guidance set forth in the Belmont Report (1979) and the Declaration of Helsinki (2024 revision).
Informed consent to participate in the study will be obtained from all participants. Additionally, because consent will be documented electronically, all participants will be given a one-page handout to keep for their records that provides most of the information contained in the informed consent with the exception that any references to alcohol will be removed to protect participants’ confidentiality should someone in the household find it.
Not applicable.
Surveys and de-identified survey data and health information (e.g., hemoglobin A1c, HIV viral load; available for South African participants only) will be made available upon request and approval of a data use agreement through the GSDG Data Library at https://www.gsdgdatalibrary.org/. Requesters may also contact the Data Library Manager, Bruce Lawrence (lawrence@pire.org).
The research and preparation of this manuscript were supported by funding from the ABIF, USA, under the contract Measurement and Evaluation—Global Smart Drinking Goals. By contract, the authors had independent decision authority on all content. The funder had no role in study design, data analysis, decision to publish, or preparation of the manuscript. The ABIF funded the development of the eSBI software and the programming of our independently developed survey consent scripts and data collection into that software. It also funded large-scale SBI/eSBI in Zacatecas-Guadalupe and Alexandra, with our study focused on the SBIs delivered from our start date until we reached our targeted sample size.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1382
Download: 109
Times Cited: 0
Mirko Casu, Pasquale Caponnetto
Mehr Muhammad Adeel Riaz ... Faisal A. Nawaz
