Affiliation:
Faculty of Health Sciences, Ontario Tech University, Oshawa, ON L1G 0C5, Canada
Email: graziella.demichino@ontariotechu.net
ORCID: https://orcid.org/0009-0009-0823-5879
Affiliation:
Faculty of Health Sciences, Ontario Tech University, Oshawa, ON L1G 0C5, Canada
ORCID: https://orcid.org/0000-0001-8608-0889
Explor Digit Health Technol. 2025;3:101163 DOI: https://doi.org/10.37349/edht.2025.101163
Received: May 08, 2025 Accepted: September 02, 2025 Published: September 27, 2025
Academic Editor: Atanas G. Atanasov, Medical University of Vienna, Austria
Aim: In 2023, the average woman used 13 different personal care products (PCPs) daily, exposing them to 114 different chemical toxins, including carcinogens, synthetic preservatives, and fragrances. Parabens, commonly used as preservatives and fragrance ingredients, are found in household and PCPs, such as cosmetics and hair products. Exposure to parabens has been associated with an increased risk of breast cancer and endocrine disorders. This study aims to evaluate the effectiveness of the “Paraben-Free & Me” educational toolkit (OSF Registration DOI: 10.17605/OSF.IO/WXU34) by exploring whether it influences changes of 3.5 points (10%) in paraben-free behaviour in women aged 18–35 years old when compared to the control group.
Methods: This study consists of a randomized controlled trial to evaluate the effectiveness of the educational toolkit among 101 female students aged 18 to 35 years old following a four-week intervention period. Baseline and follow-up questionnaires were used to assess participants’ knowledge and access to information, risk perception, health beliefs, and paraben-free behaviour by providing composite scores for each construct.
Results: The change in the knowledge and access to information score was significantly greater in the intervention group compared to the control group (+3.96, 95% CI [1.95, 5.96]). There were slight differences between groups in relation to health beliefs (+0.63, 95% CI [–1.02, 2.27]), risk perception (+0.96, 95% CI [–0.61, 2.54]), and paraben-free behaviour (–0.16, 95% CI [–2.88, 2.57]); however, these were not statistically significant.
Conclusions: This study suggests that while the “Paraben-Free & Me” educational toolkit was unsuccessful in promoting greater paraben-free behaviour in the intervention group, it can increase women’s knowledge and access to information related to parabens in their PCPs. Future studies can focus on evaluating paraben-free behaviour and the effectiveness of the educational toolkit by exploring biological measures, such as urinary concentrations of parabens, and air pollutant concentrations.
Women use an average of 13 personal care products (PCPs) daily, exposing them to 114 different chemicals [1]. One of the most used preservatives in PCPs is parabens [2, 3]. Parabens are made from petrochemicals and are used as both synthetic preservatives and fragrance ingredients, as they possess antimicrobial properties, preventing mold and bacteria growth [4]. However, chronic exposure to parabens may disrupt the endocrine system, adversely impact reproductive health, and increase the risk of breast cancer, as highlighted by several studies [5–7]. Parabens also appear in amniotic fluid and breastmilk, and they have adverse impacts on fetal development [8, 9]. There are six types of parabens typically found in PCPs: methyl-, ethyl-, propyl-, isopropyl-, butyl-, and isobutyl paraben [4]. The short-chain parabens (i.e., methyl- and ethyl-paraben) are often combined when used in PCPs, while butyl-paraben is typically found on its own [4].
Parabens are the most common preservatives used in PCPs [2]. In a study of chemicals of concern used in women’s PCPs, parabens were frequently identified on product labels, with different types of parabens usually co-occurring in the same product (49 of 81 products investigated) [3]. The use of parabens as preservatives and fragrance ingredients continues to increase [10]. According to the United States Food and Drug Administration’s Voluntary Cosmetic Registration Program database from 2019, methylparaben (MP) was reported to be used in 11,739 formulations (9,347 of which are leave-on products). This was an increase from the 8,786 uses that were reported in 2006 [10]. Propylparaben (PP) had the next highest number of reported uses at 9,034 (7,520 uses in leave-on products), which was an increase from 7,118 uses in 2006. Alarmingly, while PCP use has increased for both men and women, women are still exposed to more chemicals than men from these products (114 vs 105) [1]. Chronic daily exposure to parabens can yield high concentrations in the body [11, 12].
Concerningly, regulatory approaches to parabens in PCPs vary significantly, with stricter restrictions in Europe compared to Canada, where the use of parabens remains largely unregulated despite potential health risks. For instance, in Europe, isopropyl paraben, isobutyl paraben, phenylparaben, benzylparaben, and pentylparaben are banned from use in PCPs due to their potential endocrine-disrupting activity [13]. Furthermore, methyl-, ethyl-, and other short-chain parabens are restricted to concentrations of 0.4% for single esters and 0.8% for mixtures of esters. The Scientific Committee on Consumer Safety (SCCS) has established acceptable daily intakes (ADIs) for the differing forms of parabens [14]. MP, for example, should not be used in amounts greater than 10 mg per body weight per day. However, despite the significant health concerns associated with parabens, there are no restrictions on the use of parabens in PCPs in Canada [15]. Only products registered as Natural Health Products with Health Canada have limits to the concentration of parabens that can be used [16]. While the Government of Canada has acknowledged the potential health concerns due to parabens by completing a chemical risk assessment in 2020, no changes have been made to restrict or eliminate their use in PCPs [17]. Based on these findings and the lack of a regulatory framework on parabens in PCPs, educational resources are needed to inform women about the harmful effects of endocrine disruptors, such as parabens, allowing them to make educated decisions regarding PCP use.
Due to a lack of regulatory oversight in Canada, there is a need for behavioural interventions to address the health risks of chronic paraben exposure, especially among women who are at elevated risk [1]. Additionally, women often assume paraben-containing products are safe, as consumers are not intuitively aware of the possible risks associated with using various PCPs and share moderate concern about being exposed to endocrine-disrupting chemicals (EDCs) [18, 19]. To highlight, Trifunovski et al. [20] found that women aged 18 to 35 years old had low risk perception of parabens in PCPs, with 28% of participants having not previously heard of parabens. They also observed positive associations between high knowledge, risk perception, health beliefs, and participants’ avoidance of parabens in PCPs [20]. These findings underscore the importance of increasing women’s knowledge and access to information, health beliefs, and risk perception to promote paraben-free behaviour. In combination with the lack of a regulatory framework on parabens, women’s low risk perception and beliefs suggest a need for other types of interventions to equip them with the knowledge and skills to limit their personal exposure to parabens. The resources that are presently available, such as pamphlets, workshops, and smartphone applications, are not financially or easily accessible for users when shopping.
To help improve women’s knowledge, we designed an educational toolkit in the form of a smartphone-based application called “Paraben-Free & Me” following the Integrate, Design, Assess, and Share (IDEAS) framework for developing effective digital interventions. The goal of “Paraben-Free & Me” is to increase women’s knowledge and access to information, health beliefs, risk perception, and avoidance behaviour concerning parabens in their PCPs (paraben-free behaviour). Our primary objective is to evaluate the effectiveness of “Paraben-Free & Me” after a four-week intervention period by measuring changes in paraben-free behaviour in women aged 18–35 years old compared to the control group who did not receive the educational toolkit. Secondary objectives included comparing participants’ knowledge and access to information, health beliefs, and risk perception as measures to evaluate the effectiveness of the educational toolkit. We hypothesized that women who had access to the “Paraben-Free & Me” educational toolkit would demonstrate an improvement of 10% in paraben-free behaviour (3.5 points), knowledge and access to information (3.5 points), health beliefs (2.5 points), and risk perception (2.5 points) scores when compared with those who did not have access to the toolkit. This work is grounded in the Health Belief Model (HBM) of behaviour change, which aims to increase the likelihood of adopting positive health behaviours based on the individual’s perceived severity of disease and susceptibility of developing diseases related to parabens, and the perceived benefits of adopting the health behaviours of using paraben-free PCPs (Figure 1) [21, 22].
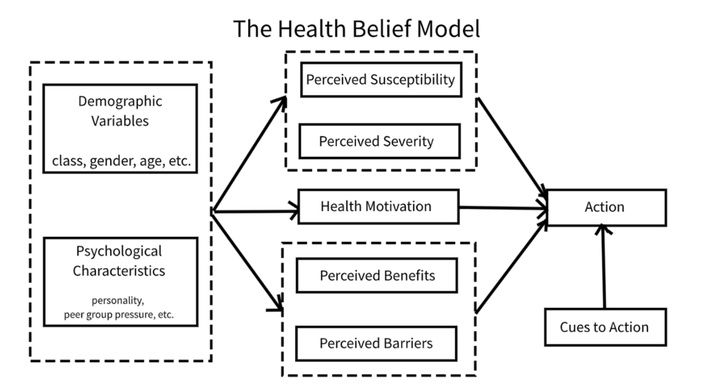
The Health Belief Model of behaviour change. Taken from [22] without modification. Accessed May 16, 2025. © Copyright-2019 Fanwang0912. Licensed under the Creative Commons Attribution-Share Alike 4.0 International license.
The effectiveness of the “Paraben-Free & Me” educational toolkit was evaluated using a two-arm randomized control trial where participants were randomized to a “Paraben-Free & Me” intervention group or a no-intervention control group, for a four-week period (OSF DOI: 10.17605/OSF.IO/WXU34). This single-site study was conducted at Ontario Tech University between January and May 2024. Data collection related to the primary and secondary outcomes took place at baseline and after the four-week intervention period. Ethics approval for this research was received from the Ontario Tech University Research Ethics Board on 05 October 2023 [REB#17494].
We recruited participants from undergraduate Health Sciences classes at Ontario Tech University via an electronic poster shared through the campus electronic learning management system. The poster contained a summary of what participation in the study entailed, as well as a link to the consent form and questionnaire. Additionally, a booth was set up in the atrium of the Health Sciences building for in-person recruitment. In order to participate in this study, participants must have met the following inclusion criteria: (1) were cisgender women (assigned female at birth), (2) were undergraduate students at Ontario Tech University, and (3) were 18 to 35 years old. Participation was voluntary, and individuals were required to provide consent before completing the questionnaire. As compensation for their time, participants received a $20 gift card to Shoppers Drug Mart, a Canadian retail pharmacy chain.
Simple randomization was used to allocate participants to either the control group or the “Paraben-Free & Me” intervention group. All participants were assigned a number between 1 and 101 at the same time, without considering any identifying characteristics or baseline variables. An automated random number generator was used to assign participants to either the control or intervention arms, ensuring allocation concealment. Participants were asked to keep their group assignment confidential to protect anonymity. No stratification was applied during randomization.
Participants randomized to the intervention group were asked to use the “Paraben-Free & Me” educational toolkit (https://www.mobileapp.app/to/KOIADJT?ref=mam). Participants in the intervention arm had access to this toolkit for four weeks, allowing them to learn about parabens, their negative health effects, what to look for in their existing PCPs, and how to find paraben-free alternatives. This also provided participants with the opportunity to use the toolkit while shopping for PCPs. Participants in the control arm did not have access to the “Paraben-Free & Me” toolkit or any other educational materials. The “Paraben-Free & Me” toolkit was designed with the aim of increasing paraben-free behaviour. It was developed in collaboration with the Women’s Healthy Environments Network (WHEN), a non-profit organization that aims to educate individuals about environmental health and prevent environmental health harms (https://www.womenshealthyenvironments.ca/). It was then pilot tested on a group of two undergraduate and two graduate students aged 18 to 35 years old, who provided feedback on the educational content, design, and layout of the toolkit. A detailed description of the “Paraben-Free & Me” toolkit and its development are included in a working paper.
The “Paraben-Free & Me” toolkit uses the HBM by targeting women’s perceived susceptibility and severity to parabens by informing them of the harmful effects of parabens through blog posts, videos, podcast recommendations, and quizzes (Table 1). It also informs them on the benefits of using paraben-free products and addresses any barriers by providing them with resources to access healthier alternatives. The “Paraben-Free & Me” toolkit provides users with a collection of resources to support learning, such as blog posts, quizzes, a forums section, YouTube videos, documentaries, and podcasts. To increase perceived severity and perceived susceptibility, the blog posts cover a variety of topics related to parabens, their negative implications, tips for shopping, and other smartphone apps that they can use (Figure 2). Perceived benefits were addressed by highlighting the advantages of switching to paraben-free products, including potential improvements in both health and appearance. The posts were multimedia, had simple, consistent, and frequent messaging, and discussed both health- and appearance-based motivators. This provided information about health consequences from credible sources [23]. Short 2–3 question quizzes were available for users to test their knowledge on parabens and to see what topics they may need to do more reading on (Figure 3). This provided users with the opportunity to test their knowledge, creating a learning experience that encourages self-rewards [23]. The app also included a forum section for users to share their knowledge and any concerns with each other, as well as provide them with the opportunity to recommend alternative products that they have personally tested, fostering an interactive learning environment that features social support (Figure 4) [23]. Finally, additional resources were available, such as YouTube videos, documentaries, and podcast recommendations, to provide participants with the opportunity to personalize their learning (Figure 5). Perceived barriers were addressed by offering users solutions, such as shopping tips, app recommendations, and access to the forums page for sharing alternative product suggestions. The “Paraben-Free & Me” toolkit was accessible to anyone with the toolkit’s login code and smart devices. It was available through the Spaces by Wix application. It was free, making it affordable for all users, and could be referenced while shopping.
Paraben-Free & Me intervention design.
| Focus of instruction | Learning goal |
|---|---|
| Parabens 101: What they are and why they’re used in cosmetics? | Knowledge |
| The truth about parabens: What’s the worst they can do? | Risk perception |
| Can switching to paraben-free personal care products improve your health? | Health beliefs |
| Are you sure of what you’re putting on your skin? Learn how to check the ingredients of your personal care products. | Knowledge |
| How to decode and understand product labels and websites for healthier choices? | Knowledge |
| How to identify paraben-free personal care products and make the switch: tips and tricks for healthier shopping? | Paraben-free PCP behaviour |
PCP: personal care product.

Example of “Paraben-Free & Me” blog posts created by the authors for users to learn about parabens, their harmful effects, and how to avoid them.

Example of “Paraben-Free & Me” weekly questionnaire created by the authors for users to test their knowledge about the content learned during the week’s blog posts.

Example of “Paraben-Free & Me” forum section created by the authors for participants to discuss their knowledge and product use with each other.

Example of “Paraben-Free & Me” additional resources for participants to supplement their learning in various formats, such as podcasts, YouTube videos, and documentaries.
Participants completed a baseline questionnaire through Google Forms that assessed their beliefs, existing knowledge about parabens, health risk perception, and paraben-free behaviour (Supplementary material). The primary outcome of the assessment was paraben-free PCP use behaviour, with secondary measures including knowledge and access to information, health beliefs, and risk perception. Paraben-free behaviour is defined as a behaviour that reduces one’s exposure to parabens from PCPs. The questionnaire began by asking participants questions related to their sociodemographic characteristics (Supplementary material). We collected data on age, ethnicity, income, birthplace, place of residence, and chemical sensitivity diagnoses from both these groups. This was used to stratify data based on any covariates. Indices for paraben-free PCP use behaviour, knowledge and access to information, health beliefs, and risk perception scales were created using the sum of scores for related items (Supplementary material). Paraben-free PCP use behaviour had a lower limit of 7 and an upper limit of 35. Knowledge and access to information had a lower limit of 7 and an upper limit of 35. Health beliefs had a lower limit of 5 and an upper limit of 25. Risk perception had a lower limit of 5 and an upper limit of 25.
This questionnaire was adapted from one developed for a previous study, which was tested for reliability using the Cronbach Alpha Test of Internal Consistency [24]. The authors administered the questionnaire to a sample of 200 women between the ages of 18 and 35 years old. The Cronbach Alpha values ranged from 0.77 to 0.93, indicating consistency among participant responses [24]. The questionnaire was modified to discuss parabens and PCPs specifically.
To evaluate the primary outcome, participants were asked a series of questions about their paraben-free behaviour (7 items; scale of 7–35). To evaluate the secondary outcomes, participants were asked a series of questions about their knowledge (7 items; scale of 7–35), risk perception (5 items; scale of 5–25), and beliefs (5 items; scale of 5–25). All outcomes were assessed using a Likert scale coding system.
Mean knowledge, health belief, risk perception, and avoidance behaviour scores from a previous study by Trifunovski et al. [20] were used to perform the power calculation and determine the sample size for this study. In this study, women scored an average of 21 ± 6 points in the avoidance behaviour section (scale of 6–30 in the original study). Based on this finding, we estimated that we would need to evaluate 100 participants to have an 80% power to demonstrate an increase of 10% (3.5 points) in the primary outcome of paraben-free behaviour. This sample size was calculated using the equation for superiority randomized controlled trials [25].
Chi-square (χ2) statistics were used to determine if there was statistical significance in the differences between the control and intervention groups in categories such as ethnicity, income, and birthplace at baseline and follow-up. Chi-square tests were also performed to determine if there were any statistically significant differences in the sociodemographic characteristics between those who dropped out and those who completed the whole study. Sensitivity analysis was performed using independent t-tests to compare the baseline paraben-free behaviour scores of those who dropped out and those who completed the entire study [26].
Likert scale responses for each item contained within each of the four constructs were used to create composite variables that were continuous in nature (knowledge and access to information, health beliefs, risk perception, and paraben-free behaviour), where a higher score indicated greater adherence to the desired behaviour. For example, a higher total score in knowledge and access to information suggested that the participant knows a lot about parabens and has access to educational resources; therefore, they would be more likely to adopt a new health behaviour. Group mean differences and 95% confidence intervals were analyzed to determine if there were differences between baseline and follow-up scores. No formal adjustments for multiple comparisons across these secondary outcomes were applied. All primary and secondary outcomes were evaluated using these same methods.
Among the 106 participants who completed the baseline questionnaire, 101 were enrolled and randomized (50 participants allocated to the control and 51 allocated to the intervention group) (Figure 6). The mean age of the sample was 21.40 ± 4.06 years. We found no significant differences in the baseline characteristics between the intervention and control groups (Table 2). This study lost 35 participants to follow-up (attrition rate of 34.7%), 21 of whom were in the intervention group and 14 in the control group. There were no significant differences in the baseline sociodemographic characteristics between the participants who completed the study and those who dropped out (Table 3). Sensitivity analysis revealed that the differences in baseline paraben-free behaviour scores were not statistically significant (dropped out = 22.12 ± 6.67, completed = 19.97 ± 5.71, p > 0.05). This suggests that attrition bias was not present.
Baseline sociodemographic characteristics (n = 101)a.
| Characteristics | Control (n = 50) | Intervention (n = 51) | Total (n = 101) |
|---|---|---|---|
| Age (years)b | 21.22 ± 4.05 | 21.57 ± 4.10 | 21.40 ± 4.06 |
| Ethnicityb | |||
| Caucasian origins | 10 (20.0) | 7 (13.7) | 17 (16.8) |
| African origins | 6 (12.0) | 5 (9.8) | 11 (10.9) |
| Asian origins | 24 (48.0) | 30 (58.8) | 54 (53.5) |
| Other origins | 10 (20.0) | 9 (17.6) | 19 (18.8) |
| Incomeb | |||
| Below/Equal LICOc | 10 (20.0) | 14 (27.5) | 24 (23.8) |
| Above LICOc | 34 (68.0) | 35 (68.6) | 69 (68.3) |
| Prefer not to say | 6 (12.0) | 2 (3.9) | 8 (7.9) |
| Place of residenceb | |||
| Greater Toronto area | 38 (76.0) | 41 (80.4) | 79 (78.2) |
| Other | 12 (24.0) | 10 (19.6) | 22 (21.8) |
| Place of birthb | |||
| Canada | 32 (64.0) | 35 (68.6) | 67 (66.3) |
| Other | 18 (36.0) | 16 (31.4) | 34 (33.7) |
| Personal chemical sensitivity diagnosisb | |||
| Yes/Maybe | 16 (32.0) | 15 (29.4) | 31 (30.7) |
| No | 34 (68.0) | 36 (70.6) | 70 (69.3) |
| Household chemical sensitivity diagnosisb | |||
| Yes/Maybe | 16 (32.0) | 20 (39.2) | 36 (35.6) |
| No | 34 (68.0) | 31 (60.8) | 65 (64.4) |
a: No statistically significant differences were reported in the baseline sociodemographic characteristics of the control and intervention groups; b: Continuous variables were reported as mean and standard deviations. Categorical data were presented as frequency and percentage; c: LICO stands for low-income cut-off.
Loss to follow-up analysis (n = 101)a.
| Characteristics | Dropped out (n = 35) | Completed follow-up (n = 66) |
|---|---|---|
| Age (years)b | 22.31 ± 4.40 | 20.91 ± 3.80 |
| Ethnicityb | ||
| Caucasian origins | 4 (11.4) | 13 (19.7) |
| African origins | 2 (5.7) | 9 (13.6) |
| Asian origins | 22 (62.9) | 32 (48.5) |
| Other origins | 7 (20.0) | 12 (18.2) |
| Incomeb | ||
| Below/Equal LICOc | 8 (22.9) | 16 (24.2) |
| Above LICOc | 25 (71.4) | 44 (66.7) |
| Prefer not to say | 2 (5.7) | 6 (9.1) |
| Place of residenceb | ||
| Greater Toronto Area | 24 (68.6) | 55 (83.3) |
| Other | 11 (31.4) | 11 (16.7) |
| Place of birthb | ||
| Canada | 23 (65.7) | 44 (66.7) |
| Other | 12 (34.3) | 22 (33.3) |
| Personal chemical sensitivity diagnosisb | ||
| Yes/Maybe | 11 (31.4) | 20 (30.3) |
| No | 24 (68.6) | 46 (69.7) |
| Household chemical sensitivity diagnosisb | ||
| Yes/Maybe | 13 (37.1) | 23 (34.8) |
| No | 22 (62.9) | 43 (65.2) |
a: No statistically significant differences were reported in the baseline sociodemographic characteristics of participants who completed the study and those who dropped out; b: Continuous variables were reported as mean and standard deviations. Categorical data were presented as frequency and percentage; c: LICO stands for low-income cut-off.
In-app analytics provided insights on intervention fidelity and user engagement. Of the 30 participants who downloaded the “Paraben-Free & Me” toolkit, 25 (83.3%) completed the course of intervention in its entirety. Blog posts were viewed frequently, with some view counts suggesting that users may have read blog posts multiple times throughout the intervention period (blog 1: 57 views, blog 2: 52 views, blog 3: 59 views, blog 4: 64 views, blog 5: 21 views, blog 6: 37 views).
Table 4 presents the group mean differences and 95% confidence intervals for the knowledge and access to information, health belief, risk perception, and paraben-free behaviour scores. The change in the knowledge and access to information score was significantly greater in the intervention group compared to the control group (+3.96, 95% CI [1.95, 5.96]). There were slight differences between groups in relation to health beliefs (+0.63, 95% CI [–1.02, 2.27]), risk perception (+0.96, 95% CI [–0.61, 2.54]), and paraben-free behaviour (–0.16, 95% CI [–2.88, 2.57]); however, these were not statistically significant.
Differences in control and intervention group means at baseline and follow-up (n = 66).
| Variable | Control (n = 36) | Intervention (n = 30) | Mean difference | 95% CI |
|---|---|---|---|---|
| Paraben-free PCP behaviour(7–35) | 3.41 (5.44) | 3.25 (5.58) | –0.16 | –2.88, 2.57 |
| Knowledge and access to information(7–35) | 0.70 (4.26) | 4.66 (3.81) | +3.96 | 1.95, 5.96 |
| Health beliefs(5–25) | 0.09 (2.43) | 0.72 (4.15) | +0.63 | –1.02, 2.27 |
| Risk perception(5–25) | 0.97 (2.50) | 1.93 (3.87) | +0.96 | –0.61, 2.54 |
Bold numbers indicate that “Knowledge and access to information” showed statistically significant differences in the intervention group compared with the control group.
The development and evaluation of “Paraben-Free & Me” have led to the creation of an evidence-based educational toolkit to help women access knowledge and increase their awareness about the health impacts of parabens in their PCPs. The application was able to increase women’s knowledge and access to information regarding the harmful effects of parabens in their PCPs and how to look for healthier alternatives.
These findings are consistent with a study by Kim et al. [27], who developed a web-based educational toolkit to reduce women’s exposure to phthalate metabolites, bisphenol A (BPA), triclosan, and parabens. The educational toolkit incorporated educational videos, games, and question and answer sections. The urinary concentration of parabens was measured before (T1), during (T2), and after (T3) the intervention. When comparing the changes in the urinary concentrations from T2 to T3 between the control and intervention groups, a statistically significant decrease was observed in mono(2-ethylhexyl) phthalate (+2.4% vs −3.8%, p = 0.013), mono(2-ethyl-5-oxohexyl) phthalate (−0.5% vs −16.3%, p = 0.046), BPA (–9.9% vs –28.4%, p = 0.038), MP (+33.6% vs –9.2%, p < 0.001), and PP (+62.2% vs –24.4%, p < 0.001).
Similarly, a study by Hagobian et al. [28] aimed to develop an educational resource to reduce bisphenol concentrations in women classified as obese with a Body Mass Index of ≥ 30.0 kg/m2. The educational toolkit consisted of weekly emailed newsletters containing educational materials and weekly in-person meetings with a counsellor to promote self-regulation and positive reinforcement. The authors measured fasting urinary bisphenols, creatinine, and weight at study entry and after 3 weeks. There was a significant treatment × time effect on creatinine-corrected urinary bisphenol S (BPS), with a decrease of 1.42 μg/g creatinine in the intervention versus 0.09 μg/g creatinine in the control group (p < 0.005) [28]. Additionally, a study by Harley et al. [29] observed that by providing an educational resource, in the form of in-person sessions and replacement products, methyl- and PP urinary concentrations decreased by 43.9% (95% CI: –61.3, –18.8) and 45.4% (95% CI: –63.7, –17.9), respectively. While these studies did not collect data on participants’ knowledge, the measures of urinary concentration can be used to imply an increase in knowledge and avoidance behaviour. By incorporating some of the same components, the “Paraben-Free & Me” educational toolkit was also able to increase users’ knowledge.
This study fills the research gap in developing an educational toolkit that educates women on the negative impact of parabens and assists them in finding healthier alternatives. In addition, this RCT evaluated the effectiveness of the educational toolkit by exploring whether it influences women’s paraben-free behaviour in relation to PCP use. There are, however, a few limitations in this study. First, data collection of knowledge and access to information, health beliefs, risk perception, and paraben-free behaviour were self-reported, which suggests there was a potential for response bias. To further evaluate “Paraben-Free & Me,” measuring biological markers such as urinary and blood concentrations of parabens, as well as air pollutant concentrations using indoor air quality monitors, can be used to support the self-reported questionnaire. Second, while 101 participants participated in the baseline data collection, only 66 completed the follow-up questionnaire. The attrition rate of this study was 34.7%, which is less than the 40% considered to be due to “fatal” flaws in the study design [30]. Furthermore, the four-week intervention period may have been too short to successfully promote paraben-free behaviour. While previous literature has suggested that a four-week intervention period aimed at increasing participants’ self-efficacy can be successful in promoting behavioural change [31, 32], varying intervention lengths may be beneficial. Third, while previous research has indicated that a four-week intervention may be successful, this shorter period could be a limitation, and longer intervention periods may be necessary. Finally, while the participants recruited for this study were from a wide range of ethnicities, incomes, places of residence, and places of birth, recruiting solely from the student population does present a limitation with the generalizability of the results. Future research should evaluate the effectiveness of the “Paraben-Free & Me” toolkit among the general public.
Despite the significant health concerns associated with parabens, there are no restrictions on their use in PCPs in Canada [15]. As a result, many products are often put on the market without rigorous testing and restrictions, leading to delayed discovery of health outcomes. Lack of awareness remains a key challenge when implementing new policies; therefore, the findings of this study may serve to generate discourse related to the lack of regulatory action by Health Canada in regard to parabens in PCPs and the importance of having educational toolkits accessible. As observed by this research, as well as the work by Kim et al. [27] and Hagobian et al. [28], educational toolkits have the potential to increase women’s knowledge about EDCs and facilitate positive behaviour changes.
Future directions include the development of the “Paraben-Free & Me” toolkit as a freestanding smartphone-based application for consumers to download from their device’s application stores. In partnership with WHEN, we hope to share the toolkit with their audiences and network. In this way, we hope to disseminate information on why parabens in PCPs are harmful and promote positive behaviours in a format that is accessible to everyone. Additionally, since the “Paraben-Free & Me” educational toolkit was developed with the general population in mind, with no gender, age, or location restrictions, future research can explore its effectiveness among different populations. With an increase in the proportion of men using PCPs, we hope to evaluate the toolkit’s effectiveness among this population in the next stage of research.
This study suggests that the “Paraben-Free & Me” educational toolkit can increase women’s knowledge and access to information related to parabens in their PCPs. Future studies could evaluate paraben-free PCP use behaviour and assess the effectiveness of the “Paraben-Free & Me” educational toolkit by evaluating biological markers or measuring air pollutant concentrations. This educational toolkit has the potential to reduce barriers for women of various backgrounds by disseminating the evidence of why parabens are harmful and what to look for in a format that is accessible and easy to understand. By reducing exposure to parabens, it encourages health improvements and enhanced quality of life.
BPA: bisphenol A
EDC: endocrine-disrupting chemical
HBM: Health Belief Model
MP: methylparaben
PCP: personal care product
PP: propylparaben
WHEN: Women’s Healthy Environments Network
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/101163_sup_1.pdf.
We would like to thank the Women’s Healthy Environments Network for their ongoing consultations throughout this project.
GDM: Formal analysis, Investigation, Methodology, Visualization, Writing—original draft, Writing—review & editing. CB: Supervision, Conceptualization, Resources, Software, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing—original draft, Writing—review & editing. Both authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Ethics approval for this research was received from the Ontario Tech University Research Ethics Board on 05 October 2023 [REB#17494] and complies with the Declaration of Helsinki.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The datasets from this study are available from the corresponding author, Graziella De Michino, upon request.
This study was funded by the SSHRC Small Grants Program 2023-Ignite Stream (Grant number not available). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1190
Download: 112
Times Cited: 0
