Affiliation:
1Ospedale Civile di Baggiovara, Department of Internal Medicine, Azienda Ospedaliero-Universitaria di Modena (–2023), 41100 Modena, Italy
†These authors contributed equally to this work.
Email: a.lonardo@libero.it
ORCID: https://orcid.org/0000-0001-9886-0698
Affiliation:
2Department of Medicine, Division of Gastroenterology, Duke University, Durham, NC 27708, USA
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0003-1824-1067
Explor Dig Dis. 2025;4:1005101 DOI: https://doi.org/10.37349/edd.2025.1005101
Received: September 26, 2025 Accepted: November 16, 2025 Published: November 26, 2025
Academic Editor: Han Moshage, University of Groningen, the Netherlands
Females are more susceptible to alcohol-related liver disease (ALD) owing to increased risk of alcohol dependence; decreased gastric first-pass effect and increased risk of producing hepatotoxic metabolites, higher alcohol bioavailability, and hormonal fluctuations affecting ethanol metabolism. Male sex is independently associated with hepatitis B virus (HBV) infection and hypertransaminasemia in HBV chronic infection. Compared to women, men have higher risks of being hepatitis B surface antigen (HBsAg) carriers, exhibit higher non-response and lower long-term immunity after prophylactic vaccination, have a higher risk of chronic hepatitis, and fibrotic and hepatocellular carcinoma (HCC). Females have higher spontaneous hepatitis C virus (HCV) clearance and reduced risk of fibrosis, cirrhosis, and HCC than men. However, post-menopausal women experience more rapid progression of hepatic fibrosis and HCC development and lower response rates to antiviral regimens compared to younger women. Hormonal and immunological mechanisms explain these sex differences observed in chronic viral hepatitis B and C. Sex and reproductive status affect the risk of metabolic dysfunction-associated steatotic liver disease (MASLD) development and progression. Genetic sex and sex hormones are involved in the pathogenesis of sex differences in MASLD by differential effects on body fat distribution, insulin sensitivity, and oxidative stress. HCC may arise as a complication of ALD, HBV, HCV, and MASLD and has a definite prevalence in the male sex because of the most robust inflammatory response of the male sex and the anti-inflammatory activity of estrogens. We conclude that those major sex differences which are identifiable in the epidemiology and clinical course of ALD, viral hepatitis owing to HBV and HCV, MASLD, and HCC. These sex disparities are explained by biological sex and sex hormones affecting metabolism, immunity, fibrogenesis, and cancer, and are the foundations for precision medicine approaches in these common hepatological conditions.
Biological sex, along with the socio-cultural construct of gender, plays a crucial role in health, disease, and medicine. Male-to-female comparisons remain a cornerstone of biomedical research, offering valuable insights into sex-based differences [1, 2]. Precision medicine aims to tailor treatments to individual patients, ensuring that the right drug is given to the right patient at the right time to optimize outcomes [3]. In this regard, the importance of relevant genetic characterization is often emphasized. However, a better understanding of sex and gender differences in each disease condition is also a critical step toward actualizing precision medicine across medical fields, notably including the hepatological arena [4–10].
Chronic liver disease (CLD) is a major contributor to global morbidity and mortality, ranking among the leading causes of death worldwide. Major CLDs, including alcohol-related liver disease (ALD) [11], infection with major hepatotropic viruses [hepatitis B virus (HBV) and hepatitis C virus (HCV)] [12], metabolic dysfunction-associated steatotic liver disease (MASLD) [13], exhibit distinct sexual dimorphisms in their epidemiological and clinical features, as well as in their underlying pathomechanisms. These disease conditions contribute significantly to hepatocellular carcinoma (HCC), the most common type of primary liver cancer, which itself exhibits major sex disparities [14].
While recent reviews have covered the spectrum of endocrine disorders and the role of sex in MASLD development and fibrotic progression [15, 16], our scoping review article has several distinctive features. We highlight updated key epidemiological findings and the underlying pathomechanisms that explain sex differences across ALD, HBV, HCV, MASLD, and HCC. Specifically, we emphasize disparities attributable to biological mechanisms spanning this spectrum of liver diseases and also discuss evolving and emerging topics to identify research gaps pertaining to sex differences.
Articles were identified through bibliographic research conducted in the PMC/PubMed database in August 2025. The search matched the keywords “sex differences” with ALD, viral hepatitis, MASLD, and HCC. All articles published in English from inception were considered for discussion, provided that both authors agreed regarding their importance. Additionally, relevant cross-references were identified, along with any pertinent articles known to the authors.
Throughout the manuscript, the terms “man” and “woman” refer to male and female humans, respectively, unless otherwise specified.
The consumption of alcoholic and alcohol-like fermented beverages has been widespread across many cultures as early as the seventh millennium BC [17]. Today, approximately 2.3 billion people worldwide consume various types of alcohol, with over half of the population in the Americas, Europe, and the western Pacific. Additionally, more than 25% of the population aged 15 to 19 are currently drinkers [18]. Despite broad awareness of its potential adverse health and social consequences, alcohol remains the most widely consumed recreational beverage, and it is frequently misused or abused as a psychoactive substance [19].
The ALD spectrum spans from alcohol-associated steatosis (affecting up to 90–95% of individuals with alcohol misuse), to steatohepatitis (in up to 35% of those with steatosis), cirrhosis (in up to 20% of individuals with steatohepatitis), and alcohol-associated HCC, which occurs in up to 10% of cirrhotic cases [11]. Acute alcohol-associated hepatitis, an acute-on-chronic liver injury with prominent cholestasis, can occur at any stage of the above-mentioned disease spectrum but is most often observed among those with cirrhosis [20].
Epidemiological and clinical data indicate that the global burden of ALD is increasing. Contributing factors include rising alcohol use among women and young people, sex-biased physiological differences in alcohol metabolism, increased alcohol consumption during the COVID pandemic, and the synergistic effects of alcohol and metabolic dysfunction, particularly in individuals with obesity [21, 22].
Strong evidence supports the notion that women are more susceptible to ALD at any given level of alcohol intake. Compared to abstainers, the relative risk of ALD is 7.3 in women as opposed to 3.7 in men [11]. The incidence of alcohol-related cirrhosis is higher in women (4.7%) than in men (3.1%), and women with ALD are generally younger and experience higher rates of complications, comorbidities, and mortality owing to alcohol-associated hepatitis [11]. Although ALD remains more prevalent in men overall, mortality from ALD is increasing more rapidly among women [11].
Sex-biased susceptibility to ALD is attributed to complex, multi-system mechanisms. Compared to men, women have an increased risk of alcohol dependence, reduced gastric first-pass metabolism, and greater production of toxic metabolites (such as acetaldehyde) owing to enhanced hepatic oxidative pathways and higher hepatic alcohol dehydrogenase activity [10]. Moreover, women generally have a higher proportion of adipose tissue, resulting in a smaller volume of alcohol distribution. As a result, the same quantity of alcohol leads to higher blood alcohol concentrations in women [11]. Finally, hormonal fluctuations unique to women (i.e., menstrual cycle) may further affect ethanol metabolism, explaining why women are more vulnerable than men to ALD [11]. Supporting this notion, female rodents also exhibit more severe alcohol-induced liver injury than male littermates [23, 24], reinforcing the notion of sex-specific susceptibility in ALD.
In either sex, estrogens are generally protective against hepatic fibrosis through inhibiting stellate cell activation and collagen synthesis [25, 26]. This protective effect has been demonstrated in experimental models as well as epidemiological research [13]. Therefore, the observation that women with ALD have worse outcomes, despite estrogen’s antifibrotic role, is paradoxical and requires additional investigation.
These female-specific vulnerabilities to ALD stand in contrast to traditional observations that women have a lower risk of cirrhosis and cirrhosis decompensation [27, 28] and tend to have better outcomes after liver resection [29]. This contrast suggests that pathomechanisms underlying worse clinical outcomes in females with ALD are distinct and etiology-specific [30].
Underlying mechanisms contributing to worse outcomes of ALD in females are likely multifactorial and complex, with dysregulation of hepatic inflammation and liver regeneration playing a significant role. Generally, males have poorer outcomes than pre-menopausal females after major liver surgery, which, however, does not necessarily imply that females have superior regenerative capacities [31, 32]. An experimental zebrafish study has found that liver regeneration, measured by hepatocyte proliferation and liver mass recovery, began earlier in males than in females after partial hepatectomy. This was linked to earlier activation of androgen receptor (AR)-regulated Yap1 signaling in male livers, while in females, estrogen receptor activation delayed the onset of hepatocyte proliferation [33]. Moreover, S100A1, a calcium-binding protein, was found to regulate the sex disparity in liver regeneration [33].
The finding that the female sex does not necessarily have superior regenerative capacities implies that additional pathobiological mechanisms may be at play. Endoplasmic reticulum (ER) also plays a critical role in liver regeneration. Experimental data suggest that the inhibition of the ER stress response after partial hepatectomy enhances liver regeneration, whereas the same inhibition after chemically induced hepatotoxicity impairs liver regeneration [34]. Sex differences in ER stress responses have been observed and are regulated by testosterone as well as estrogens. Therefore, physiological differences in sex hormones, shaped by biological sex and reproductive status, as well as their interaction with the nature of injury, may contribute to the complex sex differences observed across different liver diseases.
Beyond hepatocyte regeneration, immune and inflammatory mechanisms also likely contribute to sex differences in ALD outcomes. In the western diet alcohol mouse model of ALD, Kupffer cells exert anti-inflammatory action while infiltrating monocytes/macrophages are proinflammatory [35], underscoring the critical role of immune cells in ALD pathogenesis. Established sex-specific regulation of the immune and inflammatory responses is widely acknowledged both in extra-hepatic conditions (such as aortic stenosis, glomerulonephritis, psoriasis, and COVID-19) and in HBV infection [36]. These differences, together with specific studies [37], fully support the notion that liver-specific immune dynamics may help explain the heightened vulnerability of females to ALD. Moreover, the proliferation and differentiation of hepatic progenitor cells (HPCs) drive the homeostatic renewal of the liver under diverse conditions. Given that the endocannabinoid system promotes HPC proliferation and maturation by modulating cellular energetics [38], the characterization of sex differences in the endocannabinoid system is a key research priority.
To sum up, a better understanding of sex-biased differences in the pathobiology of ALD and mechanisms underlying female vulnerabilities in ALD is key in formulating effective interventions for women, who represent a rapidly growing subset of ALD patients [39, 40].
Significant research gaps persist regarding sex differences in ALD, particularly in understanding molecular mechanisms such as variations in adipose tissue dysfunction, alcohol metabolism, including acetaldehyde accumulation in women, and hormonal influences, especially during the post-menopausal period. Future research should prioritize the development of sex-specific clinical guidelines, address the underrepresentation of women in clinical trials of both behavioral and pharmacological interventions, and establish refined risk models that account for the accelerated disease progression observed in women. Additional investigations are also warranted into the influence of co-factors such as obesity and bariatric surgery in female populations, potential sex-based differences in hepatic encephalopathy management, and strategies to promote equitable allocation of liver transplantation resources.
HBV, a member of the Hepadnaviridae, is a small DNA virus that replicates through an RNA intermediate and can integrate into the host genome, allowing it to persist in infected cells [41]. It is estimated that two billion people have been infected with HBV globally, with 1.5 million new infections occurring each year. Approximately 300 million people are chronically infected, yet only about 10% of those individuals are diagnosed [42].
HBV infection is associated with a wide spectrum of liver disease. Acute HBV infection can be either asymptomatic or present with symptomatic acute hepatitis, a fraction of which culminates in fulminant liver failure, which will recover in most cases [41]. However, infection becomes chronic in up to 10% of cases. While many individuals with viral chronic hepatitis B (CHB) have mild liver disease not impacting long-term morbidity or mortality, others develop active chronic HBV infection, which can progress to cirrhosis and liver cancer over time [41].
The clinical course of HBV infection is strongly influenced by the mode of transmission. Perinatal (vertical) transmission leads to chronic infection in approximately 90% of cases, whereas horizontal transmission later in life results in spontaneous recovery in about 90% of individuals [43]. These contrasting outcomes underscore the importance of infection control among women as a critical step in curbing HBV transmission at the population level [44].
The male sex is an established risk factor for HBV infection and for active hepatitis [i.e., chronic abnormal alanine transaminase (ALT)] among individuals with chronic HBV infection [45, 46]. Compared to women, men are more likely to be carriers of hepatitis B surface antigen (HBsAg) [47], suggesting chronic infection, exhibit a higher rate of HBV vaccine non-response [48, 49], and have lower long-term immunity after prophylactic vaccination [50]. Men also have up to a two-fold higher risk of chronic hepatitis, hepatic fibrosis, and HCC compared to women [47, 51]. Conversely, compared to men, women tend to have lower HBV viral loads, higher HBsAg clearance rate (1.907% vs. 0.4%), and HBeAg seroconversion rate (12.9% vs. 7.71%) than men [51]. These clinically meaningful sex differences in the course of HBV infection are illustrated in Figure 1.
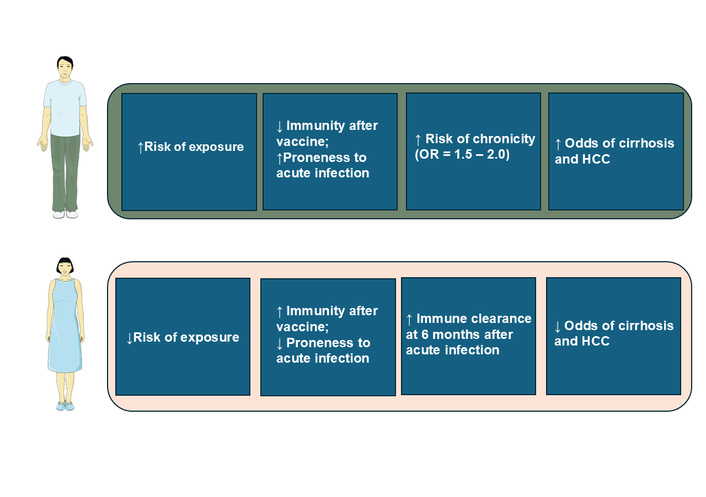
Schematic overview of sex disparities in hepatitis B virus (HBV) infection. This cartoon summarizes sex differences as a function of risk of exposure, immunity after vaccination, risk of chronicity, cirrhosis, and hepatocellular carcinoma (HCC). The figure was created with SMART - Sevier Medical Art (https://smart.servier.com/) under the CC BY 4.0 license, and was adapted from [47]. © 2022 Brown R et al. Licensed under a Creative Commons Attribution License.
As a result of these sex disparities, the male:female ratio increases markedly across stages of HBV-related disease, from 1.2 among asymptomatic HBsAg carriers to 6.3 in CHB and 9.8 in HBV-related HCC [47]. Additionally, a recent study reported that, compared to women, men had a 16% higher probability of clinical remission and 31% higher probability of attaining biochemical response after treatment with Tenofovir and Entecavir [52], two nucleoside/nucleotide analogues that inhibit HBV polymerase, thereby effectively suppressing viral replication.
Prior studies strongly suggest sex differences observed in HBV-related disease are, in part, driven by sex hormones. Compelling evidence for the roles of sex hormones in the replication of HBV includes landmark observations that adult male mice exhibit higher HBsAg titers than female mice [53]. These titers decrease following gonadectomy [54] and are restored by androgen supplementation [55].
Mechanistically, activation of the AR stimulates two androgen response elements (AREs) located in the enhancer 1 (EnhI) region of the HBV genome. This leads to increased expression of all four HBV mRNAs, thereby enhancing HBV protein production and viral replication [47]. This establishes a positive feedback loop between androgen exposure and HBV protein expression, helping to explain the higher HBV viral load and higher HBsAg titers seen in mouse males and men [47], the worse clinical outcome of HBV infection in men compared to women [12] and the correlation between serum testosterone levels and HCC risk in individuals with chronic HBV infection [47].
Conversely, activation of estrogen receptor alpha (ER-α) suppresses HBV mRNA transcription and HBV replication. This occurs through downregulation of hepatocyte nuclear factor 4-alpha (HNF4-α), a transcription factor that promotes HBV transcription by binding to the EnhI region of the HBV genome [47].
Beyond direct effects on the HBV life cycle, biological sex also influences immunological differences. Females generally mount stronger innate, humoral, and cytotoxic responses, likely due to the immunostimulatory effect of estrogen [56]. Additionally, females may have an immunological advantage through enhanced expression of toll-like receptor 7 (TLR-7), which is encoded on the X chromosome. Escape from X-inactivation or the presence of gain-of-function single-nucleotide polymorphisms (SNPs) may lead to increased TLR-7 activity in females, promoting stronger type I interferon signaling and enhancing T and B cell responses [47]. In contrast, males tend to exhibit more pronounced IL-6 responses than females, which have been associated with HCC risk [57].
The more favorable biochemical response to certain antivirals observed in males appears limited to comparisons with postmenopausal women, with no difference seen between men and women under age 50 years, presumed premenopausal. This age-specific pattern suggests that menopause-associated hormonal changes may influence treatment response to anti-HBV treatments [52]. Whether these differences reflect variations in drug metabolism or other underlying mechanisms remains unclear.
Lastly, hepatic steatosis (MASLD), whose risk is significantly influenced by sex and menopausal status, may also modulate HBV replication. In an HBV mouse model, steatosis inhibited viral replication [58]. Similarly, clinical data suggest an inverse association between HBV serum markers and MASLD [59]. MASLD may hamper HBV replication in CHB or enhance antiviral responses by activating innate immune responses [60]. Proposed mechanisms include reduced expression of peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC-1α) and increased apoptosis of HBV-infected hepatocytes [61].
Compared to evidence from animal studies, data in humans are more limited and predominantly observational in nature. Earlier menarche has been associated with earlier HBeAg seroconversion and a faster decline of HBsAg titers, supporting a potential protective effect of estrogen against HBV [47]. In contrast, earlier menarche has also been linked to an increased HCC risk among HBsAg carriers, suggesting that early estrogen exposure may not confer protection in this population [62, 63]. Postmenopausal status, however, appears to abrogate the hepatoprotective effect conferred by female sex, as sex differences diminished after the age of 50 among individuals with HBV infection [64, 65]. Consistently, the loss of protection associated with menopause can be mitigated by hormone replacement therapy (HRT), with benefits observed in proportion to the duration of treatment [63].
Existing data strongly suggest that sex and sex hormones influence HBV replication and treatment response. However, substantial research gaps remain in understanding sex differences in HBV-related liver disease in humans, particularly the specific molecular mechanisms by which sex hormones influence the HBV life cycle; the complex interplay between sex, immune response, and disease progression; the impact of life stages such as puberty and menopause on outcomes; the long-term and transgenerational effects of vertical transmission; and the extent to which lifestyle factors and disparities in healthcare access between men and women contribute to differing disease outcomes. Future studies will evaluate the impact of hypogonadism, androgen deprivation therapy, and synthetic hormone use (e.g., contraceptives, postmenopausal hormone replacement, anabolic steroids) on HBV-related liver disease and treatment outcomes. In addition, further investigation into the mechanisms underlying age-specific sex disparities in hepatitis B treatment is warranted to support the development of individualized management strategies for chronic HBV infection [66]. Furthermore, sex, reproductive status, and medical and medication histories influencing sex hormone levels should be accounted for in future guidelines, prospective studies, or clinical trials to better inform precision decision-making in HBV management.
HCV is an RNA virus belonging to the Flaviviridae family [67]. HCV is transmitted parenterally via contaminated blood and blood products, primarily through injection drug use, sexual contact, maternal-to-child transmission, and occupational exposure [68, 69]. The clinical spectrum of HCV infection is highly variable. Individuals infected at a younger age may experience little or no disease progression over time, while up to 20% of chronically infected patients develop cirrhosis within 20 years, placing them at high risk of HCC [68]. Factors accelerating adverse liver outcomes comprise older age at the time of infection, alcohol consumption, HIV or HBV co-infections, and male sex [68, 70].
Beyond hepatic involvement, HCV infection is associated with extrahepatic manifestations such as cryoglobulinemia, lymphoma, insulin resistance/type 2 diabetes, and neurological disorders have been described in association with HCV infection [71]. According to the World Health Organization (WHO), approximately 50 million people are living with chronic HCV infection globally, with an estimated 1 million new infections annually [72]. Over recent decades, the global prevalence of chronic HCV infection has declined owing to the death of chronically infected individuals and the widespread use of highly effective oral direct-acting antiviral (DAA) therapies, introduced in 2014. These treatments offer high cure rates, short durations, and minimal side effects [73].
HCV infection outcomes differ markedly by sex. A meta-analysis of 30 published studies, including 97,597 males and 96,024 females, has clearly shown that while HCV RNA seroprevalence in Egypt was similar across sexes, men had a significantly higher prevalence of HCV RNA positivity, indicating lower spontaneous clearance rates in men compared to women [74]. This supports the observation that females have a greater likelihood of spontaneous HCV clearance.
Additionally, men exhibit faster and more frequent progression of chronic HCV infection to liver fibrosis, cirrhosis, and HCC [12]. However, post-menopausal women experience a more rapid progression of hepatic fibrosis and HCC, along with lower response rates to antiviral regimens compared to younger women [12].
Sex influences both the HCV replication cycle and the host’s immune response, potentially contributing to the observed sex differences in the epidemiology of HCV infection. 17β-estradiol, via binding with the ER-α, impairs the production of mature HCV virions [12, 75]. Additionally, estradiol hinders HCV entry into hepatocytes by downregulating occludin, a key receptor used by HCV to enter hepatocytes [76]. Magri et al. [76] demonstrated in vitro that 17β-estradiol inhibited HCV infection by 64–67% (IC50 values 140–160 nmol/L), whereas testosterone or progesterone showed no inhibitory effects. Mechanistic studies revealed that 17β-estradiol exerted minimal inhibition on viral entry (< 20% inhibition of HCV pseudo-particles) and had no effects on viral RNA replication in N17/JFH1 replicon-expressing cells. In a dual-step infection model, the IC50 significantly decreased from 134 nmol/L in the primary infection to 100 nmol/L in the secondary infection (P = 0.02), with greater reductions in extracellular HCV RNA and infectivity than in intracellular viral RNA. These findings suggest that 17β-estradiol interferes with HCV assembly and/or release and partially with viral entry, but not with viral RNA replication.
Sex also affects immune responses during HCV therapy. In a study analyzing transcriptional drivers in liver samples from 195 patients undergoing HCV therapy, females exhibited stronger interferon and humoral responses, while males showed a dominant cellular immune response [77].
Finally, estradiol and intrahepatic estrogen receptors exert antifibrotic effects by protecting hepatocytes from pro-fibrogenic stimuli such as oxidative stress, inflammatory injury, and cell death [77]. Di Martino et al. [78] conducted a bi-center retrospective study supplemented by a survey including 201 women with HCV infection (43% response rate) that the rate of hepatic fibrosis progression was greater in postmenopausal than in premenopausal women (P < 0.05) and in nulliparous women compared with those who had one or multiple pregnancies (P = 0.02). Furthermore, among postmenopausal women, the estimated rate of fibrosis progression (± SE) was significantly lower in those receiving HRT compared with untreated patients (0.099 ± 0.016 vs. 0.133 ± 0.006, METAVIR units/yr; P = 0.02) and was comparable to that in premenopausal women (0.093 ± 0.012 METAVIR units/yr).
As discussed above, sex and sex hormones influence both HCV replication and host immune responses. How this evidence should inform individualized HCV treatment and management remains unclear. Existing research highlights significant gaps in understanding sex differences in HCV-related liver disease. In particular, there is a need for clinical trial data stratified by sex and for deeper insight into how hormonal changes, such as those associated with menopause, influence treatment outcomes. Furthermore, studies involving transgender populations remain scarce; their risk and outcomes for liver disease, particularly in the context of gender-affirming hormone therapy, warrant dedicated investigation. Future priorities include advancing preclinical models to better capture these distinctions and exploring the complex relationships between sex, race/ethnicity, and liver disease progression. Further investigation is needed to understand sex-biased differences in extrahepatic HCV manifestation, as well as the potential impact of synthetic hormone use, anti-estrogen therapies, and premature surgical menopause on HCV-related disease progression. These insights are essential to further advance precision medicine approaches. The development and validation of sex-specific HCV interventions should be prioritized as part of the global HCV elimination effort [79].
MASLD is defined by the presence of hepatic steatosis in individuals with at least one cardiometabolic risk factor and no other discernible cause. MASLD encompasses both uncomplicated steatosis [metabolic dysfunction-associated steatotic liver (MASL) and metabolic dysfunction-associated steatohepatitis (MASH)], histologically characterized by hepatocellular ballooning and lobular inflammation, with or without fibrosis. MASLD can progress to cirrhosis, which may sometimes lose the characteristic histologic features of nonalcoholic steatohepatitis (NASH) and is then classified as cryptogenic cirrhosis. MASLD is also increasingly recognized as a primary cause of HCC, even in the absence of cirrhosis [80].
While prevalence varies by region, MASLD currently affects more than 30% of the general population globally, and its incidence continues to rise [80]. The global prevalence of MASLD in 2021 was approximately 1.27 billion with an age-standardized rate (ASR) of 15,018.1 cases per 100,000 population, representing an 11.2% increase (95% UI, 10.5% to 11.8%) in ASRs from 2010 to 2021 [81]. The highest MASLD prevalence is observed in North Africa and the Middle East regions, and the lowest prevalence is found in high-income countries [82]. Further, the study demonstrated that in socioeconomically developed countries, food insecurity is associated with higher MASLD prevalence, whereas the opposite relationship was observed in socioeconomically less developed countries. The authors speculated that in the latter setting, limited healthcare access and underdiagnosis among populations with higher food insecurity may explain this finding. Overall, the study highlights geographical variations in the factors driving MASLD prevalence across countries.
Accumulated evidence strongly suggests that MASLD pathogenesis and disease severity are influenced by sex and sex hormones, with the latter exerting effects in a sex-specific manner. Sex differences in MASLD have been well-documented in previously published review articles; therefore, this article provides a brief synopsis along with selected updates.
A pioneering study demonstrated that risk factors associated with MASLD development are sex specific [83]. Strong epidemiological evidence supports the notion that sex and reproductive status influence the risk of MASLD development and progression (Table 1) [84–88]. Briefly, the sex-specific prevalence of MASLD is age-related, with distinct trends seen in men and women. In men, MASLD incidence rates rise from young to middle-aged adulthood, and then decline around the age of 50–60 years. In women, prevalence increases after menopause and declines after 70 years [89]. Consequently, postmenopausal women have a higher prevalence of MASLD compared with age-matched men [13]. Several sex-specific risk factors have been identified, such as serum testosterone levels (protective in men and detrimental in women) and polycystic ovary syndrome (PCOS). Furthermore, a recent study showed that dysglicaemia abolishes the protection against MASLD in young women [90], suggesting that dyslipidemia can be used to identify a high-risk individual among young women, even though premenopausal women are generally protected from insulin resistance and MASLD.
Age, sex, and reproductive status affect the risk of MASLD development and progression.
| Author, year | Method | Findings | Comment |
|---|---|---|---|
| Lonardo et al., 2015 [84] | Narrative review. | MASLD is more common in men, increases with age, and shows sex-specific differences.In men, MASLD increases from younger to middle age and starts declining after the age of 50–60 years.In women, pre-menopausal status is relatively spared by NAFLD, whereas advancing age and menopausal status independently predict MASLD. | Age, sex, and reproductive status are major modifiers of MASLD risk. |
| Balakrishnan et al., 2021 [85] | Meta-analysis of 54 studies totaling 62,239 MASLD cases, 5,428 MASH cases, and 6,444 advanced fibrosis cases. | General population: compared to men, women had a 19% lower MASLD risk (pooled RR, 0.81; 95% CI, 0.68–0.97; I2, 97.5%). A similar risk of NASH (RR, 1.00; 95% CI, 0.88–1.14; I2, 85.1%) and 37% higher risk of advanced fibrosis (RR, 1.37; 95% CI, 1.12–1.68; I2, 74.0%).Women with average ages ≥ 50 years had higher MASH risk than men (RR, 1.17; 95% CI, 1.01–1.36) and advanced fibrosis (RR, 1.56; 95% CI, 1.36–1.80; I2 = 0). | Age and sex (and, by inference, reproductive status) affect the risk of MASLD development and progression. |
| Cholongitas et al., 2021 [86] | Meta-analysis of MASLD in Europe comprising 19 studies in adults and 9 in children/adolescents. | Pooled MASLD prevalence was significantly higher in men than women (32.8% vs. 19.6%) (P < 0.01) and in obese/overweight boys than girls [32.5% (95% CI, 22.7–44.0) vs. 15.5% (95% CI, 7.6–29.0); P = 0.04]. | The prevalence of MASLD in European adults and children/adolescents with obesity or overweight is higher in males sex. |
| Wang et al., 2021 [87] | Nested case-control study of 1,861 cases and 17,664 controls in the multiethnic cohort study. | Later age at menarche was inversely associated with MASLD (Ptrend = 0.01).Parity, irrespective of number of children or age at first birth, was associated with increased MASLD risk (OR, 1.25; 95% CI, 1.05–1.48). Oral contraceptive use was also associated with increased MASLD risk (OR, 1.14; 95% CI, 1.01–1.29; duration of use Ptrend = 0.04).Oophorectomy (OR, 1.41; 95% CI, 1.18–1.68) or hysterectomy (OR, 1.33; 95% CI, 1.11–1.60) were associated with increased MASLD risk compared to natural menopause.Longer duration of MHT* was associated with increased MASLD risk (OR per 5 years of use, 1.08; 95% CI, 1.01–1.15). | Menstrual, reproductive factors, and use of exogenous hormones modify MASLD risk. |
| Riazi et al., 2022 [88] | Meta-analysis of 72 studies totaling 1,030,160 subjects from 17 countries. | Overall, MASLD prevalence and incidence were significantly (P < 0.0001) higher in men than in women, respectively [39.7% (36.6–42.8) vs. 25.6% (22.3–28.8) and 70.8 cases per 1,000 person-years (48.7–92.8) vs. 29.6 cases per 1,000 person-years (20.2–38.9)]. | Overall, the prevalence of NAFLD is significantly higher in men than in women. |
*: only estrogen therapy; CI: confidence intervals; MASH: metabolic dysfunction-associated steatohepatitis; MASLD: metabolic dysfunction-associated steatotic liver disease; MHT: menopause hormone therapy; NAFLD: nonalcoholic fatty liver disease; NASH: nonalcoholic steatohepatitis; OR: odds ratio; RR: risk ratio.
Sex and sex hormone status can modify the effects of genetic risk factors. A study in a nonalcoholic fatty liver disease (NAFLD) cohort provided a proof of concept by demonstrating significant gene-sex and gene-menopausal state interactions in NAFLD-related hepatic fibrosis [91]. Furthermore, a recent study on the patatin-like phospholipase domain-containing protein 3 (PNPLA3) gene—where the p.I148M variant is linked to increased liver fat content and disease severity—found that carriage of the p.I148M variant conferred a greater increase in risk across the entire spectrum of SLD in women than in men [92]. The same study also demonstrated functional interplay between PNPLA3 and ER-α, identifying an ER-α-binding site within a PNPLA3 enhancer, and showed induction of PNPLA3 by ER-α agonists [92].
The effects of PNPLA3 appear to be enhanced among older subjects. A recent study reported that patients with higher genetic risk exhibited greater increases in liver stiffness with each 10-year increase in age, and that carriers of the PNPLA3 G/G genotype experienced a more pronounced increase in liver stiffness after age 40. Moreover, the protective effects of the loss-of-function variant HSD17B13 rs72613567 on steatohepatitis and fibrogenesis vary by patient age, BMI, PNPLA3 genotype, and sex [93]. Interestingly, a more pronounced protective effect was observed among women aged 51 years or older compared with other groups, although limited statistical power within subgroups precluded clear assessment of age or age-sex interactions [93]. Collectively, these findings encourage further investigation into the biological interactions that modify the effects of genetic variants, thereby informing the individualized application of genetic information [94].
Sex differences in cardiovascular disorders (CVD) are widely appreciated [1], as is the fact that CVD is the leading cause of death among those with MASLD [95]. Consistently, sex differences in CVD risks within the MASLD population are increasingly recognized [96]. A recent Iranian study of 446 patients (59.2% female, average age 52.9 years) found that subclinical coronary artery disease risk, assessed by coronary calcium score, was independently predicted by age and diabetes mellitus in women, and diabetes and MASLD in men for single-vessel disease. For multiple-vessel disease, predictors in women include age and dyslipidemia, while in men they include age, diabetes mellitus, chronic kidney disease, and MASLD [97]. Of concern, a meta-analytic review of thirty-six cohort published studies, totaling ~18.5 million individuals (~25% with MASLD; 48% women; mean age of 50.2 years) followed for a median of 6.9 years found that, compared to men, women with MASLD were at higher risk of incident fatal and non-fatal CVD events, especially individuals with more severe MASLD [98]. These data support the implementation of sex-specific algorithms of CVD risk assessment and sex-differentiated management strategies in MASLD.
The second leading cause of mortality in MASLD is cancer [99]. Beyond the risk of HCC, which is discussed under section HCC, MASLD patients are also at increased risk for various extrahepatic cancers, notably including colorectal cancer, breast cancer in women, and pancreatic carcinoma [100, 101]. Interestingly, sex and fibrosis differentially affect the risk of HCC versus extra-hepatic cancers in MASLD. Liver fibrosis is associated with an increased risk of HCC but not extrahepatic cancers [100]. Moreover, the relative risk of developing liver cancers in the MASLD vs. non-MASLD population was 3.16 for males and 1.25 for females, while the relative risk of developing extrahepatic cancers was 1.01 for males and 1.44 for females. The relative risk ratio for females vs. males was 1.44, indicating that females have a 44% higher risk of developing extrahepatic cancers in MASLD vs. non-MASLD compared to males [100].
Pathogenesis of sex differences in MASLD is complex and mechanism-specific, and it has been increasingly reviewed in the literature. Thus, this article focuses on a broader overview.
Both sex chromosomes and sex hormones contribute to these sex differences. For example, studies using mouse models that distinguish gonadal from chromosomal effects have shown that X-chromosome dosage influences food intake, adiposity, and metabolic dysfunction, comprising insulin resistance, dyslipidemia, and SLD [102]. In humans, the XXY genotype associated with the Klinefelter syndrome (KS) exhibits a higher estradiol-to-testosterone ratio compared to XY control men; symptoms of hypogonadism and dysmetabolic comorbidities, including the metabolic syndrome, diabetes, and cardiovascular diseases, increase with advancing age [103]. Moreover, KS males have mild liver dysfunction, reflected by a significant increase in liver markers and decreased albumin; the effect of testosterone replacement therapy on these parameters remains unknown [104]. Tuner syndrome subjects have androgen deficiency [105], increased prevalence of SLD and liver fibrosis compared to healthy controls [106].
A recent meta-analysis demonstrated that menopause is associated with approximately 2.4 times higher odds of MASLD [107]. In a separate study conducted in China, which aimed to develop a validated MASLD prediction model specifically targeted at postmenopausal women who experienced natural menopause, several variables such as occupation, total and visceral adiposity, number of abortions, anxiety, metabolic factors, and diet were identified as independent predictors of ultrasound-based MASLD diagnosis among postmenopausal women [108]. Another study targeting individuals aged 50 or older raised concern that postmenopausal women with visceral obesity may have comparable or worse liver and cardiometabolic profiles than men, despite appearing healthy by standard clinical measures. These findings underscore the importance of early diagnosis and management in this specific population. Standard biomarkers, including transaminases and fatty liver index (FLI), are less reliable for detecting MASLD in women, highlighting the need for sex-specific diagnostic criteria and greater focus on postmenopausal women with central obesity for early MASLD detection and management [109].
Androgens and estrogens are present in different concentrations in both sexes, and variations in their physiological concentrations are associated with MASLD development and progression.
Estrogens regulate glucose homeostasis and lipid metabolism, suppress inflammation, and promote hepatocellular regeneration [13]. The increased risk for NAFLD among post-menopausal women, estimated to be about 2.4-fold higher [107], is likely attributable to the loss of physiological estrogens [110]. Moreover, the risk of liver fibrosis correlates directly with the duration of estrogen deficiency, as indicated by premature menopause or time from menopause [111].
Additionally, the estrogen pathway interacts with genetic variants and the hepatic transcriptome, influencing the pathogenesis and natural history of MASLD and MASH. Effect modification of genetic variants by age, sex, and sex hormones is discussed in the section on Risk factors for MASLD. A recent study comparing the liver transcriptomes of sham-operated female control mice, ovariectomized female control mice, and liver ER-α knockout (LERKO) sham-operated female mice demonstrated that hepatic ER-α drives masculinization of the liver transcriptome after menopause [112]. Moreover, as sex-specific growth hormone secretion patterns, key regulators of the hepatic transcriptome, become less pronounced in women after age 50 [113], hepatic gene expression profiles may undergo substantial shifts in postmenopausal women. These findings underscore the importance of accounting for biological variability related to menopause when designing gene expression studies.
Androgens also play a major role in liver metabolism, and perturbations in their signaling pathways may result in MASLD. In male rodents, androgens protect against insulin resistance and SLD, while in female rodents, they have steatogenic effects [13]. Androgen deficiency in men [114] and androgen excess in women [115] are risk factors for NAFLD development and progression [110]. Sex-specific androgen effects on hepatic lipid metabolism have been reviewed extensively [116]. Furthermore, men with chronic spinal cord injury have a high prevalence of hypogonadism, affecting over 40% of patients. In this population, the risk of NAFLD was independently associated with lower serum testosterone levels [117], with a proportional correlation between gonadal hypofunction and NAFLD risk. Indeed, the odds of having MASLD increased by 1% for each decrement of 1 ng/dL of total testosterone and of 3% for each decrement of 1 pg/mL of free testosterone, after adjustment for confounders [117]. These findings further underscore the clinical importance of diagnosing and managing hypogonadism in men to prevent MASLD progression.
Progesterone’s biophysiological effects are diverse, including metabolism, cellular energy homeostasis, hepatic inflammation, and immunity, in addition to its reproductive functions [118–120]. Progesterone stimulates the deposition of body fat and exerts catabolic effects on protein metabolism. The association between MASLD and adverse pregnancy outcomes is likely multi-factorial [121].
Weaning, rather than delivery, terminates the metabolic effects of pregnancy in mothers. Further to MASLD development, lactation protects from the fibrotic evolution of progression of MAFLD and MASH via high prolactin levels. Details are reviewed elsewhere [122].
Sex differences in MASLD are increasingly recognized in both experimental and clinical research, reflecting contributions from sex chromosomes, sex hormones, and sex-specific epigenetic profiles. Key research gaps related to sex differences in MASLD include the development of sex-specific diagnostic criteria, a more comprehensive understanding of hormonal influences, encompassing both physiological and synthetic hormone use, and how these factors modulate disease progression and immune responses in MASLD. Further investigation is also warranted to elucidate sex-based differences in the effects of genes such as PNPLA3 [123], contributions to cardiovascular mortality, and the development of targeted therapeutic strategies. A deeper understanding will require a comprehensive characterization of sex-related transcriptomic signatures in both diseased and healthy livers. Future studies should systematically account for sex, age, and reproductive status across all stages of research—from cell lines, organoids, and animal models to observational studies and clinical trials. Integrating these insights through multidisciplinary collaboration will accelerate the development of precision medicine strategies for MASLD prevention, diagnosis, and treatment.
According to a global study using data in 2012, chronic infection with HBV contributes to 44% of all HCC cases worldwide, followed by alcohol consumption (26%), HCV (21%), cigarette smoking (13%), and obesity (9%) [124]. In a U.S. cohort study of 181,346 participants followed over a median of 23.1 years, 753 incident HCC were identified. Population attributable risks (PARs) were highest for obesity (14.5%; 9.2–19.8%), lower coffee intake (21.3%; 95% CI: 8.9–33.0%), current smoking (15.1%; 11.1–19.0%), heavy alcohol use (7.1%; 3.5–10.6%) and lower diet quality (4.1%; 0.1–8.1%) [125].
In a U.S. study of 319 HCC patients and 1,061 healthy controls, regular cigarette smoking was associated with HCC in men (AOR, 1.9; 95% CI, 1.1–3.1), while heavy alcohol consumption was associated with HCC in women (AOR, 7.7; 95% CI, 2.3–25.1). Synergistic interactions were observed between cigarette smoking and chronic HCV infection in men (AOR, 136.3; 95% CI, 43.2–429.6) and between cigarette smoking and heavy alcohol consumption in women (AOR, 13.7; 95% CI, 3.2–57.9) [126].
In a large study using the Surveillance, Epidemiology, and End Results (SEER)-Medicare linkage, 10,708 HCC cases from the SEER database were compared to a 5% random sample of cancer-free persons (n = 332,107) to compute population attributable fractions (PAFs). The highest PARs were for metabolic disorders (32.0%), HCV (20.5%), alcohol use (13.4%), smoking (9.0%), HBV (4.3%), and genetic disorders (1.5%) [127].
As discussed above, the relative contributions vary according to the regional prevalence of viral infections. However, these findings underscore the substantial role of MASLD and other metabolic disorders in HCC pathogenesis. This topic has been reviewed in detail elsewhere [128].
Globally, HCC tends to be more prevalent in males than in females [129], with disease rates among men being three-fold higher than in women [130]. The male:female ratio is 5 in France and 4.8 in Malta [129], and lowest in Costa Rica (1.6), Colombia, Ecuador, and Uganda (1.3 for all) [130]. Geographic variability in the male:female ratio likely reflects the interplay of multiple behavioral and cultural (i.e., gender-related, such as smoking and alcohol), viral, hormonal, and metabolic factors influencing HCC pathobiology [9].
Beyond these epidemiological differences, recent analysis has highlighted substantial sex disparities in the natural course and treatment outcomes of HCC. Compared to females, males exhibit higher HCC-related mortality and a greater risk of late recurrence following liver resection or ablation. In contrast, women tend to present with HCC at an older age and at earlier stages, leading to better overall survival rates. This survival advantage in women is age-dependent and disappears after the age of 60 [131]. Moreover, liver transplantation in females is associated with lower disease recurrence and reduced mortality compared with men [131].
A complex array of sex differences in the disease pathobiology explains the variability in the epidemiological features and clinical outcomes of HCC.
After exposure to di-ethyl-nitrosamine, male mice show a significantly higher response in the pro-inflammatory, tumor growth- and metastasis-promoting factor IL-6, which acts through the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway. Consistently genetically engineered Knock-out mice exhibit a reduced incidence of HCC and longer survival than wild-type animals [132]. At the same time, estrogen decreases the expression of TNF-α, another pro-inflammatory cytokine capable of activating cancer cells through the NF-κB signaling pathway [133]. Moreover, estradiol exerts direct anti-fibrotic action in a mouse model of fibrosis induced by carbon tetrachloride [26].
In male hepatocytes derived from patients with HCC, the AR promotes the proliferation and transformation of liver tumor cells through activation of Wnt/β cyclin signaling [134] and increased expression of c-Myc, an important regulator of cell proliferation and survival [135].
Conversely, in females, estrogen inhibits HCC cell proliferation via the ER-α receptor, upregulates the tumor suppressor protein tyrosine phosphatase receptor type O (PTPRO), and reduces the activity of the transcription factor STAT3 through modulation of the PI3K and JAK pathways [135]. Estrogens also block the proliferation of cancer cells and promote cell apoptosis by increasing cell cycle proteins P21 and P27 [136]. Additionally, ER-β can downregulate peroxisome proliferator-activated receptor α (PPARα) and its downstream genes by binding to the EREs within the PPARα gene, thereby inhibiting HCC development.
Regarding the effects of sex hormones on intrahepatic target cells, estrogens promote the synthesis of endothelial nitric oxide synthase (eNOS) and NO in sinusoidal endothelial cells and DNA synthesis in hepatocytes while blocking the ROS production and the IL-6 secretion in Kupffer cells and Type I collagen synthesis and alpha-smooth muscle actin (alpha-SMA) deposition in hepatic stellate cells [131]. In contrast, androgens enhance the mitogenic activity in hepatocytes, promote angiogenesis in sinusoidal endothelial cells, increase IL-6 secretion in Kupffer cells, and stimulate Type I collagen synthesis and alpha-SMA deposition in hepatic stellate cells [131].
As evidence for sex-specific HCC pathobiology emerges, more studies have examined sex differences in HCC treatment efficacy. Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of HCC. In this context, sex-based differences in their efficacy are increasingly recognized [137]. However, the data remain controversial, highlighting the complexity of this topic and supporting the necessity of additional investigation [138–140]. Current research gaps regarding sex differences in HCC encompass elucidating the specific molecular mechanisms that contribute to observed disparities in HCC and its metastatic propensity [14], optimizing immunotherapy approaches according to sex, and advancing diagnostic and screening techniques for all patients. Additional areas of research include sex differences in response to and survival after surgical treatments. Furthermore, it is essential to integrate sociocultural factors into clinical practice. Additional studies are warranted to clarify the inconsistent findings related to the progression of advanced CLD to HCC. Finally, incorporating sex and sex hormone status in clinical trials of HCC treatment would enhance our knowledge and help advance individualized HCC treatment strategies in the future.
Major sex differences are evident in the epidemiology and clinical course of ALD, viral hepatitis owing to HBV and HCV, MASLD, and HCC. These sex disparities are accounted for by biological sex and sex hormones affecting metabolism, immunity, fibrogenesis, and cancer (Figure 2). Several translational implications arise from this field, ranging from sex-specific predictive models for hepatic outcomes and extra-hepatic (CVD, cancer) events. From the therapeutic perspective, sex-specific precision medicine approaches may include estrogen replacement therapy, anti-androgenic treatment, as well as innovative tailored pharmacological interventions targeting hormonal pathways [13, 141]. Beyond biological sex, additional risk modifiers, including lifestyle factors (alcohol consumption, dietary habits, smoking, and physical activity), adiposity, pharmacological exposures, and others, collectively referred to as “cofactors” [142], contribute independently and interact synergistically to the development of CLD. However, these factors are often challenging to accurately capture, standardize, and compare across therapeutic studies. Finally, clinical trials addressing sex differences, along with reproductive status, are of vital importance for avoiding any sex bias in hepatology while supporting evidence-based strategies of tailored treatment in men and women [143, 144].
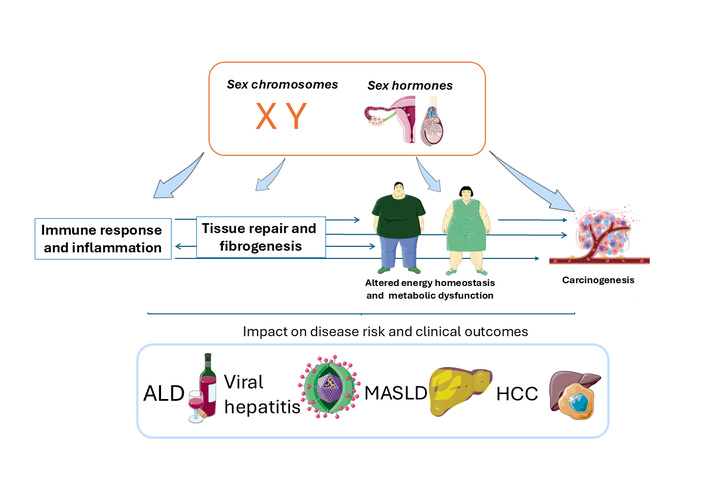
Pathomechanisms of sex differences in chronic liver disease (CLD). Schematic overview of the principal modifiers (clockwise from top right): sex chromosomes and hormones, metabolic dysfunction, immunity, hepatic fibrogenesis, (hepatic and extrahepatic) cancer, involved in the development and progression of the most common forms of liver disease (central cartoon). ALD, viral hepatitis owing to hepatitis B virus (HBV) and hepatitis C virus (HCV), MASLD, and HCC. ALD: alcohol-related liver disease; MASLD: metabolic dysfunction-associated steatotic liver disease; HCC: hepatocellular carcinoma. The figure was created with SMART - Sevier Medical Art (https://smart.servier.com/) under the CC BY 4.0 license.
ALD: alcohol-related liver disease
AR: androgen receptor
ASR: age-standardized rate
CHB: chronic hepatitis B
CLD: chronic liver disease
CVD: cardiovascular disorders
EnhI: enhancer 1
ER: endoplasmic reticulum
ER-α: estrogen receptor alpha
HBsAg: hepatitis B surface antigen
HBV: hepatitis B virus
HCC: hepatocellular carcinoma
HCV: hepatitis C virus
HPCs: hepatic progenitor cells
HRT: hormone replacement therapy
KS: Klinefelter syndrome
MASLD: metabolic dysfunction-associated steatotic liver disease
NAFLD: nonalcoholic fatty liver disease
PARs: population attributable risks
PNPLA3: phospholipase domain-containing protein 3
PPARα: peroxisome proliferator-activated receptor α
SEER: Surveillance, Epidemiology, and End Results
TLR-7: toll-like receptor 7
AL and AS contributed equally: Conceptualization, Investigation, Methodology, Project administration, Validation, Writing—original draft, Writing—review & editing. Both authors read and approved the submitted version.
Amedeo Lonardo, who is the Associate Editor and Guest Editor of Exploration of Digestive Diseases, had no involvement in the decision-making or the review process of this manuscript. Ayako Suzuki declares no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
