Affiliation:
Department of Health Information Technology, University of Maryland, Baltimore County, Baltimore, MD 21250, United States of America
Email: km11737@umbc.edu
ORCID: https://orcid.org/0009-0001-3244-9609
Explor Digit Health Technol. 2026;4:101177 DOI: https://doi.org/10.37349/edht.2026.101177
Received: July 10, 2025 Accepted: November 12, 2025 Published: January 03, 2026
Academic Editor: Sil Aarts, Maastricht University, Netherlands
Vaccines have eliminated once-deadly diseases, yet rising vaccine hesitancy threatens these gains. Human papillomavirus (HPV) illustrates this crisis: Although it is one of the few vaccines that directly prevents cancer, uptake remains low in the United States and globally, particularly in regions with high cervical cancer incidence. This persistent gap undermines both individual and public health. This paper examines how digital health technologies, aligned with policy frameworks and community engagement, can address HPV vaccine hesitancy. We propose the Digital Vaccine Advocacy Toolkit, a structured, HPV-focused framework that integrates electronic health record (EHR)-based clinical decision support, personalized reminders, population dashboards, AI-driven misinformation surveillance, and culturally tailored education. As a conceptual model, it draws on secondary evidence and policy recommendations rather than original empirical data, emphasizing interoperability, privacy safeguards, equity-driven design, and stakeholder engagement to support feasibility across diverse health systems. The Toolkit is organized into illustrative workflows that demonstrate how technical features could be combined with policy mechanisms and financing models to strengthen HPV vaccination. By situating HPV within the World Health Organization’s 90-70-90 elimination targets and the recent adoption of single-dose schedules, the framework highlights both translational relevance and global applicability, though its recommendations require pilot testing and empirical validation. Overall, the Digital Vaccine Advocacy Toolkit offers a practical roadmap for improving HPV vaccine uptake through the integration of technology, policy, and ethics, and provides a transferable model for advancing digital health strategies to increase vaccine confidence and equity in immunization programs worldwide.
Human papillomavirus (HPV) vaccine hesitancy illustrates how misinformation, stigma, and systemic inaction converge to undermine even cancer-preventing interventions. Although safe and effective against cervical cancer and other HPV-related diseases, uptake remains low [1]. Substantial declines in cervical precancers among young women since vaccine introduction emphasize its impact [2, 3]. Gendered misinformation has framed HPV as solely a women’s issue, leaving many men unprotected against HPV-related cancers [4]. HPV strains also cause anal and oropharyngeal cancers in both sexes, genital warts, and recurrent respiratory papillomatosis (RRP), a rare condition that impairs breathing [5]. These outcomes show HPV’s broad risks and the urgency of equitable vaccination strategies.
Despite this urgency, health systems underuse digital tools that could improve uptake. A meta-analysis found electronic medical record (EMR)-based interventions increased HPV vaccine initiation and completion by 4–7% [6], while a U.S. border clinic showed EMR prompts nearly doubled immunization odds and boosted completion by over 10 points [7]. These results demonstrate feasibility but also fragmentation: Effective tools exist but remain scattered. This paper addresses that gap by proposing a Digital Vaccine Advocacy Toolkit to unify such approaches for HPV.
Framing HPV hesitancy within wider vaccine refusal shows why integration matters. Vaccine hesitancy, delaying or refusing vaccines despite availability, poses an escalating threat to public health. Smallpox eradication illustrates vaccination’s power when coverage is broad. Persistent gaps in vaccine uptake, the share of individuals who accept vaccination when offered, continue to hinder disease prevention, including cervical cancer [8]. These gaps stem less from scientific limitations than from misinformation, politicization, and eroding public trust [9]. Sociodemographic factors such as education, employment, geography, and political identity further drive inequities [9–11]. The COVID-19 pandemic intensified these dynamics, politicizing health information and accelerating coverage declines [12, 13].
While this research highlights the complexity of vaccine hesitancy, it also exposes a critical gap: Limited attention has been paid to how digital health infrastructure can directly address HPV-related barriers. The central question posed in this paper is whether digital health technologies, such as electronic health records (EHRs), telehealth, and artificial intelligence (AI)-powered surveillance, when integrated with policy frameworks and community engagement, can form a structured HPV-focused Digital Vaccine Advocacy Toolkit. Positioning HPV within this lens highlights broader system-level shortcomings in vaccine implementation, including policy inertia, fragmented health communication, and underuse of digital innovation. Addressing HPV vaccine hesitancy requires an integrated, data-driven approach that leverages health information technology (Health IT) tools alongside policy and community engagement. This perspective outlines a scalable, Health IT-enabled framework, centered on digital tools, public policy, and community engagement, to counter vaccine misinformation, improve HPV vaccine uptake, and inform broader public health strategy.
The significance of HPV vaccine hesitancy becomes clearer when considered alongside other vulnerable populations. Patients with immune-mediated conditions such as systemic lupus erythematosus (SLE) and systemic sclerosis (SSc) face heightened infection risk, like influenza and pneumococcus. Despite proven vaccine benefits, hesitancy persists due to concerns about immune response and disease flares [14]. This pattern illustrates how even effective vaccines face uptake barriers in high-risk groups, emphasizing the need for tailored advocacy strategies for HPV. To operationalize this vision, the following sections explore how Health IT interventions, including EHR-based clinical decision support (CDS), mobile applications, telehealth, and AI surveillance, can enhance HPV vaccine uptake.
EHRs, digital systems that store and share patients’ health information, are essential for improving vaccine uptake. During COVID-19, EHRs, mobile apps, and analytics expanded coverage and enabled real-time monitoring [15]. These systems store up-to-date patient data and, when paired with CDS, which provides patient-specific recommendations, prompt providers to recommend the HPV vaccine at the right time. EHRs also track vaccination history, flag missed doses, and send automated reminders via portals, text, or email [16]. Randomized controlled trials confirm that EHR-based interventions improve HPV vaccination initiation, completion, and follow-up [6].
Emerging ambient AI tools that “listen” to provider-patient encounters could extend CDS by generating real-time prompts and adapting messaging to patient demographics. Early studies show promise, but validation and large-scale deployment remain limited [17]. Scaling these tools requires interoperability standards such as Health Level Seven International (HL7) and Fast Healthcare Interoperability Resources (FHIR). These enable data exchange across platforms and support population-level tracking.
Lenert et al. [18] showed how extending Bulk FHIR improved access to state immunization registries for coordinated outreach, yet integration remains uneven in under-resourced systems, particularly in low- and middle-income countries (LMICs) and rural areas where infrastructure and workforce capacity are limited. Ensuring compliance with established privacy regulations like the Health Insurance Portability and Accountability Act (HIPAA) in the United States and the General Data Protection Regulation (GDPR) in Europe remains essential, as these frameworks protect patient confidentiality while allowing data exchange for public health purposes [19–21].
EHR-based CDS reminders are highly scalable, as they integrate across health systems and adapt to local HPV vaccination guidelines. A New York City trial showed EHR reminders improved immunization opportunities, demonstrating feasibility for wider implementation [16]. When enhanced with analytics, these systems can also monitor HPV vaccine efficacy by linking records with later diagnoses of HPV-related disease. This strengthens confidence in its cancer-preventing benefits and helps identify gaps in coverage and patient behavior. Together, EHR/CDS interventions, augmented by ambient AI and supported by interoperability, function as infrastructure-level enablers of equitable HPV vaccine delivery.
Mobile health (mHealth) applications are accessible tools for delivering accurate vaccine information. They offer schedules, education, and links to trusted providers, helping patients navigate HPV vaccination decisions [22]. Evidence consistently links these tools with improved HPV awareness and vaccine adoption [23].
When paired with telehealth services, mHealth extends reach through real-time provider-patient engagement. Telehealth, the remote delivery of care through digital platforms, is especially valuable in underserved regions where healthcare access is limited [24]. Virtual consultations allow providers to address misinformation, discuss HPV vaccine safety, and deliver tailored recommendations that sustain engagement and reduce stigma [25].
The COVID-19 pandemic demonstrated scalability: Both mHealth and telehealth sustained immunization campaigns when in-person visits collapsed, proving feasibility for HPV vaccination in constrained settings [15]. HPV-specific digital tools, such as a culturally U.S.-Mexico border program, improved provider communication and uptake in Hispanic communities, where uptake is historically lower [26]. While promising, outcomes vary by population due to differences in digital literacy and access [27].
Together, these tools function as more than communication channels; they operate as equity-focused infrastructure, reducing barriers, expanding reach, and building continuity of care, while laying the groundwork for advanced AI and natural language processing (NLP) systems to further optimize delivery and misinformation surveillance.
AI, which enables computers to perform tasks requiring human intelligence, and NLP, which allows computers to understand and analyze human language, can be applied to monitor vaccine-related discourse online. For HPV, AI-driven tools are especially valuable for identifying persistent myths, such as the misconception that the vaccine is only relevant for women, and detecting emerging narratives that discourage uptake. These systems can flag misinformation and trigger timely responses from public health authorities [28, 29].
Practical applications are already visible: Platforms such as X (formerly Twitter) have introduced community-driven fact-checking [30]. Beyond detection, AI can perform sentiment analysis, helping public health organizations understand vaccine-related perceptions and tailor outreach to HPV-specific concerns. HPV studies using NLP on X (2008–2017) tracked sentiment shifts and false versus verified claims, showing misinformation spreads faster and engages more users; Instagram analyses found similar patterns, highlighting surveillance challenges [31, 32].
To ensure reliability, AI and NLP models are evaluated with metrics such as accuracy, precision, recall, F1-score, and area under the receiver operating characteristic curve (AUC). Transformer models like BERT and RoBERTa have been applied to HPV misinformation detection, with RoBERTa embeddings and logistic regression achieving AUC 0.958 and F1 0.88. Large-scale analyses of 650,000 HPV-related tweets achieved 71.4% recall and 31.2% precision when classifying concerns [33]. These benchmarks are vital for reducing misinformation and misclassification of marginalized voices, showing that HPV surveillance has moved from concept proposals to measurable, real-world applications.
Ultimately, AI’s impact lies not in detection alone but in integration: Misinformation insights can feed into public health dashboards, guide provider scripts, and inform school-based campaigns, transforming raw surveillance into coordinated HPV-focused responses.
Addressing HPV vaccine hesitancy also requires strong policy action. Financial constraints remain a significant barrier. Sustained vaccination requires coordination across governments, providers, schools, and civil groups to ensure scalable, culturally relevant interventions [34]. A rural U.S. study found that public health and education leaders identified school-based promotion programs and cross-sector collaboration as key opportunities to increase HPV vaccination uptake and overcome systemic barriers [35]. Free or subsidized vaccinations in schools and clinics, supported by national programs and Gavi, are critical for access and sustainability [36, 37].
Gavi’s co-financing model promotes gradual country ownership while maintaining support until programs are self-sustaining, a feature especially vital for LMICs with weak insurance systems and limited resources [37]. Gavi-supported countries have demonstrated notable increases in HPV coverage through program introduction into national schedules [38].
Legislative measures like school-entry HPV vaccination and insurance mandates for preventive vaccines can further reduce inequities and institutionalize access [39]. However, mandates often face political resistance, and financing mechanisms are shaped by broader political economies, which can affect implementation. Linguistic and cultural barriers require attention; multimedia materials and interpreter services can improve outreach to immigrant and minority communities [40]. School-based delivery with sustainable financing and community partnerships is often the most practical starting point, especially in resource-limited settings.
Structural reforms lay the foundation for equity, but policy must also address the digital influences on vaccine perceptions.
Social media is a powerful vehicle for misinformation [29]. Regulatory bodies such as the Federal Trade Commission (FTC) have issued enforcement orders requiring transparency in how platforms filter health-related ads and deceptive content [41]. The FDA has proposed draft guidance allowing “tailored responsive communications” to address third-party misinformation on medical products [42]. These steps show that voluntary measures are inadequate; binding standards like content labeling, algorithm transparency, and user education tools are needed to counter false narratives. Yet, policy reforms alone are insufficient if communities carry deep-seated mistrust shaped by historical and cultural experiences.
Mistrust in the medical system, particularly among communities of color, cannot be ignored. Historical injustices like the Tuskegee experiment have left lasting scars [43]. Digital health interventions must therefore be coupled with culturally tailored communication and meaningful community engagement to rebuild trust. This also means ensuring that emerging surveillance tools are deployed ethically, with safeguards that protect privacy and equity.
While monitoring misinformation online is an essential component of modern vaccine policy, the privacy of users must be carefully protected [44]. Clear data-use guidelines are needed to limit intrusion and prevent misuse, and algorithmic bias, such as marginalized communities being unfairly flagged, must be addressed because these disparities can reinforce existing inequities in vaccine access and trust [45]. Real-world cases, such as healthcare prediction tools underestimating Black patients’ needs, show how bias can perpetuate inequities unless systems are designed with equity and auditability from the start [46]. If similar biases emerge in digital surveillance tools, they risk undermining trust in HPV vaccination efforts, particularly among marginalized communities. Implementing safeguards such as transparency in AI decision-making, independent audits of surveillance tools, and mechanisms for community oversight are key strategies for addressing these challenges [47]. Balancing surveillance with human rights ensures that digital interventions remain trustworthy and equitable. Governance mechanisms such as algorithmic audits and community advisory boards can help ensure transparency, equity, and accountability in digital surveillance tools.
Global commitments are already underway to improve HPV vaccine uptake and eliminate cervical cancer through the World Health Organization’s (WHO) 90-70-90 strategy: By 2030, 90% of girls under 15 years old should be fully vaccinated, 70% of women screened by ages 35 and 45 using high-performance tests, and 90% of women diagnosed with cervical disease should receive appropriate treatment [48]. Achieving these targets will require leveraging digital health implementation frameworks, as the WHO has emphasized that robust health information systems are essential for tracking coverage, sending reminders, and enabling population-level data analytics to ensure accountability and equitable progress [49].
WHO has also endorsed a single-dose HPV vaccine schedule, which substantially reduces logistical and financial barriers to vaccination [50]. This shift is especially critical in LMICs where resource constraints and weak follow-up systems make multi-dose schedules difficult. LMICs, which bear nearly 90% of the global cervical cancer burden, stand to benefit most from simplified regimens, digital tracking tools, and community-based delivery approaches that improve completion rates and reduce inequities [49, 51]. However, implementation remains uneven, particularly in LMICs and rural areas where digital infrastructure and provider capacity are limited. Together, these efforts show how technology, paired with global policy, can drive sustainable and equitable HPV elimination [51].
Building on these insights, the proposed Digital Vaccine Advocacy Toolkit operationalizes these technological, policy, and equity strategies into a coordinated framework to maximize HPV vaccine uptake.
To maximize impact, a Digital Vaccine Advocacy Toolkit is proposed. This framework integrates technical infrastructure, policy, equity-focused implementation, and ethical safeguards to strengthen HPV vaccine confidence and uptake.
CDS alerts embedded in EHRs prompt timely HPV vaccine recommendations at the point of care. For scale, these alerts must be interoperable across health systems, leveraging exchange standards such as HL7 and FHIR. Personalized reminders delivered through SMS, email, or mobile applications extend this reach by tailoring notifications to patients’ vaccine schedules, including both multi-dose and single-dose regimens.
Population dashboards aggregate vaccination data to track coverage and identify gaps, with cloud-based infrastructures enabling population-level analytics while maintaining compliance with privacy regulations. Complementing these tools, AI-powered misinformation surveillance employs NLP to detect emerging narratives online. Reliability requires validation metrics (accuracy, precision, recall, F1-score, and AUC), and fairness safeguards reduce risks of algorithmic bias. Finally, multilingual and culturally tailored education delivered through mHealth platforms ensures inclusivity.
Sustained impact requires policy support. Financing models such as national immunization budgets, Gavi co-financing mechanisms, and public-private partnerships with telecoms provide long-term sustainability. Legislative measures, including school-entry vaccination mandates, insurance coverage for preventive vaccines, and platform accountability for misinformation, reinforce equitable access. Ministries of Health embed interventions into national immunization schedules, while non-governmental organizations (NGOs) and community groups support cultural tailoring and last-mile delivery.
Adaptation to context is central. In LMICs, low-bandwidth SMS systems, simplified single-dose tracking, and community health worker dashboards are emphasized. In high-income countries, advanced features such as interoperable EHR/CDS integration and app-based reminders can be prioritized, illustrating adaptability across health systems. Workflow integration creates synergy. Provider prompts trigger reminders that feed into dashboards, misinformation surveillance informs campaigns, and monitoring data guides policy decisions.
Future pilots should incorporate stakeholder feedback and cost modeling, apply implementation science methods to optimize workflow integration.
Effectiveness should be assessed using clearly defined equity-sensitive indicators with baseline comparators and data sources specified, including vaccine initiation and completion rates across demographic groups; provider adoption of CDS alerts; reductions in inequities; and shifts in digital sentiment around HPV vaccination. Using validated tools such as standardized immunization registries and NLP sentiment analysis models can anchor these indicators in measurable outcomes.
Finally, ethical guardrails are essential. Patient data must be managed under HIPAA, GDPR, or equivalent standards. AI tools should undergo independent audits to detect and mitigate algorithmic bias, with transparency in decision-making to ensure accountability. Community oversight mechanisms can further align digital strategies with public expectations, sustaining trust and preventing unintended harms.
Together, these elements form a scalable, ethically grounded framework that positions digital health as a catalyst for sustained HPV vaccine confidence and equity. Figure 1 illustrates the workflow of the Digital Advocacy Toolkit for HPV vaccination, showing how clinical, data, and communication interventions are integrated with ethical safeguards across all components.
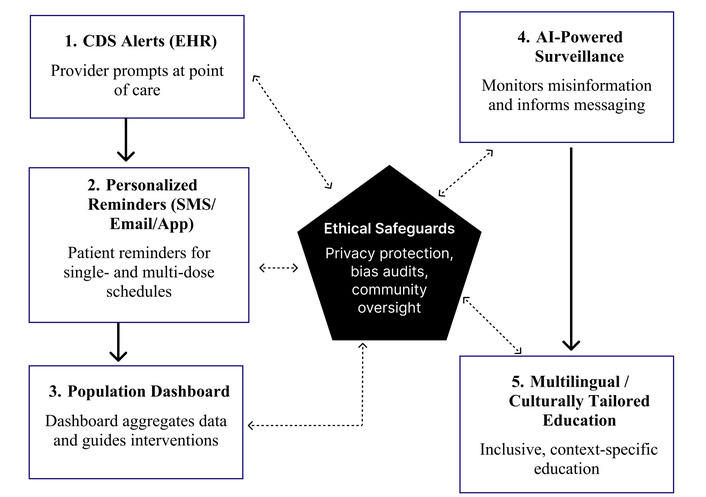
Workflow of the Digital Vaccine Advocacy Toolkit for HPV vaccination. The framework integrates provider prompts, patient reminders, and dashboards (clinical/data flow) with AI surveillance and inclusive education (communication/education flow). Ethical safeguards, including privacy protection, bias audits, and community oversight, apply across all components to ensure equity and trust. CDS: clinical decision support; EHR: electronic health record; AI: artificial intelligence; HPV: human papillomavirus.
This paper introduces the Digital Vaccine Advocacy Toolkit as a novel, structured framework that integrates technical, policy, and ethical dimensions to address HPV vaccine hesitancy. Unlike broader proposals, the Toolkit is HPV-focused, technically detailed, and operationalized with clear pathways. By uniting EHR prompts, telehealth, AI misinformation monitoring, and financing models into a coherent structure, it provides a practical roadmap for high- and low-resource settings.
Its contribution lies not in listing interventions, but in weaving them into a system-level translation strategy that balances scalability with ethical safeguards and equity-driven design. The inclusion of WHO’s 90-70-90 elimination targets and the single-dose HPV vaccine schedule further situates the framework within global elimination efforts.
Future steps include piloting the Toolkit in diverse health system contexts, empirically testing its impact on HPV vaccine uptake, and evaluating policy pathways that enable scale-up. If implemented effectively, the Toolkit offers a blueprint not only for improving HPV vaccination but also as a transferable model for digital health strategies in other immunization programs worldwide.
This perspective has several limitations. First, it is a conceptual paper that relies primarily on secondary sources rather than original empirical data. While the framework is grounded in existing literature and global policy recommendations, it has not yet been validated through pilot studies. Second, the proposed Digital Vaccine Advocacy Toolkit is intended as a flexible model, and its effectiveness will depend on local health system capacity, resources, and sociocultural context. Certain components, such as AI-powered misinformation surveillance, are still technically evolving and may raise concerns about equity, privacy, and feasibility in low-resource settings. The proposed evaluation metrics lack baseline comparators or statistical validation; future studies must demonstrate measurable impact. Comparative evaluations between HPV-specific interventions and other immunization programs could further clarify the Toolkit’s broader applicability. Although this paper focuses on HPV vaccine hesitancy, broader applications of the framework to other immunization programs remain to be fully explored. Future research should prioritize pilot implementations with rigorous study designs to empirically validate the framework across diverse contexts. Caution is warranted in generalizing from HPV to other vaccines, which may face distinct behavioral, logistical, and policy challenges. Despite these limitations, the Toolkit provides a foundation for future research, testing, and adaptation in diverse settings.
AI: artificial intelligence
AUC: area under the receiver operating characteristic curve
CDS: clinical decision support
EHRs: electronic health records
EMR: electronic medical record
FHIR: Fast Healthcare Interoperability Resources
GDPR: General Data Protection Regulation
Health IT: health information technology
HIPAA: Health Insurance Portability and Accountability Act
HL7: Health Level Seven International
HPV: human papillomavirus
LMICs: low- and middle-income countries
mHealth: Mobile health
NLP: natural language processing
WHO: World Health Organization
The author thanks Dr. Charles Bieberich, who taught genetics and emphasized HPV vaccination as a cancer prevention tool. Deep gratitude is extended to Professor Paul Mulhern, Health IT and Policy instructor at the University of Maryland, Baltimore County, whose guidance inspired the exploration of policy’s role in public health through informatics, and who reviewed the final manuscript draft. The author also thanks Dr. Erin Van Dyke, department head, for feedback on the early draft, and Professor Amanda Williams, whose Process and Quality Improvement course shaped the implementation pathways discussed. Finally, appreciation goes to Hero-Godsway Zilevu for assistance with figure design.
PIA: Conceptualization, Investigation, Methodology, Writing—original draft, Writing—review & editing, Project administration. The author read and approved the submitted version.
The author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2026.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2026. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1041
Download: 39
Times Cited: 0
