Affiliation:
1Liver Unit, Hospital Clinic of Barcelona, August Pi i Sunyer Biomedical Research Institute (IDIBAPS), 08036 Barcelona, Spain
2Department of Medicine and Research Laboratory of Internal Medicine, National Expertise Center of Greece in Autoimmune Liver Diseases, General University Hospital of Larissa, 41110 Larissa, Greece
3European Reference Network on Hepatological Diseases (ERN RARE-LIVER)
ORCID: https://orcid.org/0000-0002-0278-3518
Affiliation:
1Liver Unit, Hospital Clinic of Barcelona, August Pi i Sunyer Biomedical Research Institute (IDIBAPS), 08036 Barcelona, Spain
3European Reference Network on Hepatological Diseases (ERN RARE-LIVER)
ORCID: https://orcid.org/0009-0008-0766-6864
Affiliation:
1Liver Unit, Hospital Clinic of Barcelona, August Pi i Sunyer Biomedical Research Institute (IDIBAPS), 08036 Barcelona, Spain
3European Reference Network on Hepatological Diseases (ERN RARE-LIVER)
ORCID: https://orcid.org/0000-0002-3189-7557
Affiliation:
2Department of Medicine and Research Laboratory of Internal Medicine, National Expertise Center of Greece in Autoimmune Liver Diseases, General University Hospital of Larissa, 41110 Larissa, Greece
3European Reference Network on Hepatological Diseases (ERN RARE-LIVER)
ORCID: https://orcid.org/0000-0001-7075-8464
Affiliation:
1Liver Unit, Hospital Clinic of Barcelona, August Pi i Sunyer Biomedical Research Institute (IDIBAPS), 08036 Barcelona, Spain
3European Reference Network on Hepatological Diseases (ERN RARE-LIVER)
Email: mlondono@clinic.cat
ORCID: https://orcid.org/0000-0002-6533-1586
Explor Dig Dis. 2024;3:92–106 DOI: https://doi.org/10.37349/edd.2024.00042
Received: August 17, 2023 Accepted: January 14, 2024 Published: April 10, 2024
Academic Editor: Kenichi Ikejima, Juntendo University Graduate School of Medicine, Japan
Autoimmune hepatitis (AIH) is a chronic liver disease of unknown aetiology that can lead to end stage liver disease if left without treatment. Corticosteroids with or without azathioprine (AZA) are considered the recommended standard first-line treatment option for the induction and maintenance of remission. The aim of treatment is to achieve complete biochemical response (CBR), defined by normal transaminases and immunoglobulin G (IgG) within 6–12 months after treatment initiation. However, response rates to standard treatment vary widely as approximately 10–25% of cases develop intolerance, insufficient response, or rarely non-response to AZA. Mycophenolate mofetil (MMF) is an effective and safe alternative first-line treatment in AIH, based on its high rates of CBR among treatment-naive patients, but can also be considered as second-line drug in patients with poor response or intolerance to AZA. However, even after the administration of second line treatment there is a small proportion of patients with refractory disease that bear the highest probability of developing decompensated cirrhosis and hepatocellular carcinoma. For this difficult to treat subgroup of patients third-line treatments are warranted. Therefore, the aim of this review is to summarize the current evidence on second- and third-line therapies for AIH, as well as, to set the background for future perspectives on safer and more efficient treatment strategies.
Autoimmune hepatitis (AIH) is a chronic liver disease of unknown aetiology affecting all ages and races, characterized by a distinct increase of immunoglobulin G (IgG) levels in most cases, circulating autoantibodies, interface hepatitis on liver histology and favourable response to immunosuppression [1–5]. Corticosteroids with or without azathioprine (AZA) are considered the recommended standard first-line treatment option, although this recommendation is still based on trials conducted in the past five decades with the last one being published more than 20 years ago [6–11]. Thus, corticosteroids remain the drug of choice for the induction of response and AZA the preferred drug for maintenance of response. Although there is still some debate regarding the optimal dose of both drugs, international guidelines recommend the use of 0.5–1 mg/kg per day of predniso(lo)ne for the induction of response, while AZA seems better to be added 2 weeks after treatment initiation if total bilirubin is below 6 mg/dL and be titrated up to 1–2 mg/kg per day [2, 4, 5, 9, 12, 13].
The aim of treatment is to achieve complete biochemical response (CBR), defined by normal transaminases and IgG within 6–12 months after treatment initiation [5, 14]. Ideally, the treatment should also aim for histological remission [modified histological activity index (mHAI) < 4] at 12 months after treatment initiation or at any other time point during treatment, but a follow-up liver biopsy to confirm remission at the histological level is routinely not performed in everyday clinical practice [2, 4, 14]. Response rates to standard treatment vary widely from 25–80% [15–19]. However, a systemic review, including all randomized trials published since 1950, reported CBR rates of about 43%, while in a recent study with real world data from UK the on-treatment response rates ranged between 55% and 62% [20, 21]. In addition, approximately 10–25% of cases develop intolerance, insufficient response, or rarely non-response to AZA [2, 4, 5, 22], while a relapse of the disease seems almost universal after treatment withdrawal even after two or more years of sustained CBR [23].
It is therefore clear that, there is a considerable proportion of patients with refractory disease who have also the highest chance of developing long-term complications such as decompensated cirrhosis or hepatocellular carcinoma [2, 4, 5, 14, 16, 19]. For these patients second- and third-line treatments are warranted. Unfortunately, third-line therapies in AIH are not well established and are mainly based on centre experience and small case series without control groups. So, the aim of the present review is to summarize the current evidence on second- and third-line therapies for AIH, as well as, to set the background for future perspectives on safer and more efficient treatment strategies for difficult to treat patients.
The use of 6-mercaptopurine (6-MP) in AIH is basically reserved for patients with AZA intolerance which is defined as any adverse event possibly related to the treatment as assessed by the treating physician leading to potential discontinuation of the drug [14]. As mentioned above, more than 25% of AIH patients experience side effects from AZA, leading to drug discontinuation in most of them. According to a recent position statement on second- and third-line treatments [24], the use of 6-MP may be attempted, as 50–75% of patients intolerant to AZA are able to adequately tolerate 6-MP. However, this option has not been endorsed by the recent the American Association for the Study of Liver Diseases (AASLD) clinical practice guidelines [2] and other centres, as this recommendation was mainly based only on a small retrospective study with 40% of patients achieving CBR (8 out of 20 patients) [25]. The recommended initial dose of 6-MP ranges from 0.5–1.0 mg/kg per day and in case of good tolerance the titration should be according to the 6-thioguanine nucleotides (6-TGN) blood levels which however, is not widely available in everyday practice [4, 25].
Mycophenolate mofetil (MMF) is the first selective, potent, reversible, and non-competitive inhibitor of type II isoform of inosine-5’-monophosphate dehydrogenase discovered more than 100 years ago, exerting antiviral, anti-fungal, antibacterial, anti-tumor, and immunosuppressant properties [26]. As a result, MMF leads to selective immunosuppressive effect by depleting guanosine-triphosphate specifically in activated B- and T-cells without affecting the type I isoform and therefore, resulting in low rates of adverse events [5, 26]. The administration of MMF is principally justified as second-line treatment in patients intolerant to AZA according to the European Association for the Study of the Liver (EASL) and AASLD clinical practice guidelines as well as a recent position statement [2, 4, 24], even though recent systematic reviews and meta-analysis from the Australian Liver Association Clinical Research Network [27] and US [28] showed that MMF led to response rates ranging from 32.0–57.1% in insufficient responders to AZA and 62–82% in intolerant patients.
However, some national guidelines propose the use of MMF as an alternative first-line agent based on its high rates of CBR among treatment-naive patients and its low rates of adverse events and/or intolerance (< 10%) [5]. Indeed, the Hellenic Association for the Study of the Liver (HASL) has included since 2019 [5], apart from AZA, MMF as an acceptable first-line treatment option for induction and maintenance of response in AIH patients. The latter recommendation was based on several prospective cohort studies and a recent meta-analysis which showed that MMF could be an alternative and safe first-line treatment option for AIH patients [29–32].
Additionally, a recent strict prospective propensity matching trial of MMF versus AZA as first-line treatments, found that MMF was associated with higher rates of CBR bearing a higher overall efficacy than AZA, even when patients who were intolerant or unresponsive to AZA were included in the analysis [33]. Regarding the long-term response, a recent study from the same group with 21 years of follow-up, has shown lower primary non-response rates at week 4, lower rates of intolerance and serious adverse events, and more frequent eligibility to stop immunosuppression compared to those treated with standard of care in MMF group compared to the AZA group, while 78% of treatment-naive MMF-treated patients-maintained remission off prednisolone [22]. Furthermore, results from the unique controlled randomised trial of AZA versus MMF (CAMARO trial) [34] for the induction of response in treatment-naive AIH patients showed very recently, superiority of MMF to achieve CBR at 6 months (72.2% vs. 32.3%; P = 0.004) with significantly lower adverse events [35]. Taking together, these results indicate that the new guidelines will probably change in the near future both for first- and second-line treatments.
Up to the present, several studies have evaluated the efficacy of MMF in patients with AZA intolerance/insufficient response and/or as first-line option in treatment-naive patients (Table 1). Most of these studies have shown high rates of CBR, which in most cases are comparable or even better than those reported with AZA, ranging from 60–97% [22, 29–31, 33, 35–44]. On the contrary, two older studies have reported lower rates of complete response or even treatment failure following MMF initiation [45, 46]. The latter findings could be attributed to the heterogeneity of the study populations and the variability of study protocols (combination or not with corticosteroids, corticosteroids dose, duration of treatment, etc.).
Characteristics of studies reporting treatment with MMF for naive, intolerant, or refractory AIH
| Author | Year | Type | Population (N) | Follow up (months) | Patients | Response rates |
|---|---|---|---|---|---|---|
| Richardson et al. [36] | 2000 | Retrospective | 7 | 46 | 2 | 71% |
| Devlin et al. [42] | 2004 | Retrospective | 5 | NA | 1 | 100% |
| Czaja et al. [45] | 2005 | Retrospective | 8 | 19 | 2 | 0% |
| Chatur et al. [37] | 2005 | Retrospective | 11 | 26.5 | 0 | 64% |
| Hlivko et al. [29] | 2007 | Retrospective | 29 | 26 | 2 | 84% |
| Inductivo-Yu et al. [44] | 2007 | Retrospective | 15 | 41 | 2 | 73% |
| Hennes et al. [46] | 2008 | Retrospective | 36 | 49 | 2 | Naive 43%Refractory 25% |
| Sharzehi et al. [38] | 2010 | Retrospective | 21 | 6 | 2 | Naive 88%Refractory 0% |
| Zachou et al. [30] | 2011 | Prospective | 59 | 26 | 0 | 59% |
| Baven-Pronk et al. [39] | 2011 | Retrospective | 45 | 37 | 2 | Naive 67%Refractory 13% |
| Zachou et al. [31] | 2016 | Retrospective | 109 | 67 | 0 | 72% |
| Efe et al. [43] | 2017 | Retrospective | 121 | 62 | 2 | Naive 92%Refractory 34% |
| Nicoll et al. [27] | 2019 | Retrospective | 105 | 32 | 2 | 60% |
| Giannakopoulos et al. [41] | 2019 | Retrospective | 22 | 71 | 2 | 45% |
| Dalekos et al. [33] | 2022 | Prospective | 64 | 45 | 0 | Naive 97%Refractory 100% |
| Dalekos et al. [22] | 2022 | Retrospective | 183 | 66 | 0 | 96% |
| Kolev et al. [40] | 2022 | Retrospective | 50 | 24 | 2 | Naive 82%Refractory 30% |
NA: not applicable; 0: naive/intolerance; 1: refractory; 2: both
To sum up, MMF seems an effective and safe alternative first-line treatment in AIH but can also be considered as second-line drug in patients with poor response or intolerance to AZA [22, 24, 35].
Third-line treatments should be considered in patients who are adherent to therapy and in whom the disease is active and progressive despite intensified first- and/or second-line therapy. Several agents have been used as third-line treatment so far, but all of them are in small study cohorts or case series according to the experience and expertise of each centre and most importantly without disease control group (Table 2).
| Agent | N of studies | Patients | Proposed dose | Comments |
|---|---|---|---|---|
| TAC | 8 | 2 | 0.1 mg/kgTrough levels: 1–10 ng/mL |
|
| Cyclosporine | 6 | 2 | 2 mg/kg per dayTrough levels: < 120 ng/mL |
|
| Infliximab | 1 | 1 | 5 mg/kg on week 0, 2, 6 and every 4–8 weeks thereafter |
|
| Everolimus | 1 | 2 | Trough levels: 3–6 ng/mL |
|
| Sirolimus | 2 | 1 | Trough levels: 10–20 ng/mL |
|
| MTX | 1 | 1 | 10 mg/week |
|
| Rituximab | 3 | 2 | 1,000 mg on days 0, 14 and repeat if necessary, according to liver biochemistry |
|
| Belimumab | 2 | 1 | 10 mg/kg at days 0 and 14 and every 28 days thereafter |
|
DILI: drug induced liver injury; MTX: methotrexate; TAC: tacrolimus; 0: naive/intolerance; 1: refractory; 2: both
TAC is a calcineurin inhibitor with greater immunosuppressive activity than cyclosporine. A number of studies have been conducted in AIH, evaluating the use of TAC in the setting of treatment failure, incomplete response, and AZA intolerance. In all of them TAC has been administered in combination with corticosteroids and AZA or MMF. Biochemical response rates vary amongst different studies probably because of the difference in dose and trough levels. One multicentre study reported biochemical response (normalization of transaminases) in 94.1% of patients who were intolerant to standard treatment and 56.5% in those who had insufficient response to previous therapy [43]. The respective results in the same study for MMF administration were 91.9% and 34% [43]. Based on these results the AASLD recommended both MMF- and TAC-based therapies as appropriate second-line treatments [2].
Other older and smaller studies observed improvement in liver tests in most of the patients, but without achieving CBR [37, 47–52]. Regarding safety, dose reduction or discontinuation occurred in approximately 25% of cases and the most frequently reported side effects were neurological symptoms and mild increase in creatinine levels, none of these leading to treatment discontinuation [48, 49, 52, 53].
However, EASL, HASL, the European Reference Network on Hepatological Diseases (ERN RARE-LIVER), and the International Autoimmune Hepatitis Group (IAIHG) do not recommend TAC as a potential second-line option in AIH patients [4, 5, 24] because the abovementioned large study by Efe et al. [43] was a retrospective analysis of very heterogeneous group of patients treated with multiple treatment schedules and with irregular follow-up data. Recently, there are several similar studies in the literature, with more systematic data collection [54–56]. The feeling of these studies is that calcineurin inhibitors are very potent drugs but require close monitoring of drug levels because of a quite small therapeutic window while toxicity remains considerable. Actually, a recent two-centre study confirmed the limited effectiveness and the risks of this kind of therapy in AIH [57]. In the previous study [43], only neurotoxicity was mentioned, but renal injury, diabetes mellitus, hypertension, hyperlipidaemia, and malignancies also should be mentioned. Therefore, as in other immune mediated diseases, MMF can be tried in all AIH patients who are intolerant/insufficient responders to thiopurines and third-line treatment with TAC must be administered in cases with ongoing insufficient response. According to experts’ opinion, standardization of the dose so as to achieve higher initial trough levels (6–8 ng/mL) and tapering of trough levels after response has been achieved is strongly recommended [24].
Experience in the use of cyclosporine comes mainly from the paediatric population, where cyclosporine is principally used in severe disease or to prevent steroid side effects. Evidence of cyclosporine in adults with drug intolerance or insufficient response to first- or second-line treatments comes from old and small case series with biochemical response (normalization of transaminases) rates around 80% [58–60]. However, these numbers change when patients who are treatment intolerant and non-responders are simultaneously analysed. In the most recent study, including larger number of patients, response rate was 60% [61]. Therefore, it is clear that the limited number of patients and the heterogeneity of the cohorts preclude the extraction of safe conclusions.
Taking into consideration the abovementioned data, cyclosporine remains a potential third-line option in AIH. As far as the treatment schedule, recent suggestion by the ERN RARE-LIVR and IAIHG is the use of cyclosporine at a dose of 2 mg/kg per day twice daily and serum trough levels < 120 ng/mL [24] but renal function should be assessed prior to and during treatment.
TNFa inhibitors may also have a therapeutic role in AIH, although still their use remains a matter of debate. In a small single-centre retrospective study from Germany, infliximab was administered in 11 difficult to treat AIH patients including seven patients with established cirrhosis. The dose used was 5 mg/kg at day 0, week 2, and week 6 and every 4–6 weeks thereafter according to laboratory and clinical evolution of each patient. Among patients included, 6/11 (54%) achieved CBR [62]. However, a considerable number of patients (7/11, 64%) developed infectious complications needing hospitalization, while treatment had to be withdrawn in 3 cases because of side effects [62].
Although this study supports the role of anti-TNFa in the pathophysiological basis of AIH management, it is well known that TNFa blockade can sometimes result in exactly the opposite outcome inducing an immune-mediated liver disease resembling AIH or even true AIH [63–67]. Indeed, a large international pharmacovigilance database including 389 patients with anti-TNFa blockade-associated AIH, reported that infliximab was the most frequently associated drug [68]. In other words, the use of TNFa-blockade drugs seems like a “two-edged sword” management because of the unforeseen safety of these agents. This therapeutic paradox is mainly attributed to the disruption of the regulatory role of TNFa signalling throughout the immune system. Therefore, the AASLD guidelines have considered infliximab as a drug with definite association with AIH development [2].
Considering the above-mentioned data, infliximab may do have a role in the management of refractory AIH, however due to the probability of unforeseen complications more evidence regarding its efficacy and safety are warranted.
Everolimus and sirolimus inhibit the mammalian target of rapamycin (mTOR) modulating the proliferation and survival of activated lymphocytes. The use of both drugs is better studied for preventing rejection in solid organ transplantation. The experience of their use for poor responders to standard treatment in AIH is very limited.
Only one report is available about the use of everolimus in AIH, which consists of a retrospective analysis of 7 patients with intolerance or insufficient response to second- and third-line (calcineurin inhibitors) agents. All patients had improvement of liver biochemistry with trough levels ranging between 3–6 ng/mL, while 3/7 of patients achieved CBR after 5 months of treatment with no severe side effects [69].
The efficacy of sirolimus in AIH has been reported in only 5 AIH patients refractory to AZA and MMF. CBR was achieved in 2/5 of patients with serum trough levels between 10–20 ng/mL, while side effects occurred in 2/5 of patients [70]. However, a report of two additional cases of refractory AIH was more disappointing as in one case sirolimus was discontinued due to side effects, while in the second case biochemical response was not achieved [71].
So, based on these data the use of mTOR inhibitors in AIH is not recommended, until more data regarding their use are collected.
MTX is a folic-acid antagonist used mainly in the treatment of rheumatic diseases such as rheumatoid arthritis and lupus, but also as anti-neoplastic drug. Data on efficacy and safety of MTX in AIH patients are limited to case reports and one case series [72, 73]. The unique case series published from Haridy et al. [74], reports the use of MTX in 11 patients with AIH previously unresponsive to first-line treatment. The authors reported CBR in 54.5% of patients treated with MTX at a median dose of 10 mg/week during 36 months of follow-up as well as, a significant reduction of corticosteroids dosage [74]. However, nearly half of the patients (5/11, 45.5%) had to stop treatment during the first 12 months because of an elevation in liver enzymes, which in two cases occurred during the first 3 months after treatment initiation and was considered as DILI mediated by MTX [74].
So, MTX could serve as third-line treatment in AIH, but we need more data for clear-cut recommendations. In addition, strict surveillance is recommended since current data on safety and efficacy are extremely limited and there is a high risk of adverse events including the probability of DILI development.
Rituximab is a monoclonal antibody directed against B-cell surface receptor CD20. The use of rituximab for refractory AIH was first reported in a case of a 68-year-old woman with Waldenström macroglobulinaemia and diagnosis of AIH with poor response to induction treatment with corticosteroids. Rituximab was used for the induction of response which was afterwards maintained with low dose corticosteroids in combination with AZA for a long follow-up period of 4 years [75]. In a single centre open label pilot study from Canada, including 6 patients with either refractory disease (3/6) or intolerance to first-line treatment (3/6), rituximab was used with good tolerance and rapid improvement of liver biochemistry and IgG in all subjects by 12 weeks of drug infusion. Biochemical response and B cell depleting effect were maintained throughout 72 weeks of follow-up [76]. Moreover, recent results from the IAIHG in 22 patients with AIH refractory to second- and/or third-line therapy showed significant improvement of transaminases that was maintained during the 24 months of follow-up. In addition, both prednisone doses and flares were reduced (71% of patients without disease flare at 2 years) [77]. However, CBR (normalization of both transaminases and IgG levels) was not achieved perhaps because only two doses, 2 weeks apart, were administered to the patients.
According to these data, rituximab seems to be a safe and effective treatment option for refractory AIH in combination with standard treatment. The recommended dose based on published data for adults is 1,000 mg at week 0 and 2, to be repeated afterwards according to liver biochemistry (response guided). Serious adverse events seem to be rather infrequent, however, surveillance of B-cells depletion (particularly CD20 cells) is recommended as well as supplement with immunoglobulins when it is considered necessary [24].
B cell activating factor (BAFF) is a cytokine belonging to the TNF family expressed by T and dendritic cells and is proven to be crucial for the development and differentiation of B cells. BAFF levels have already been shown to be associated with liver inflammation and to be reduced in AIH patients after response to corticosteroids. Thus, inhibition of BAFF could be a pathogenetically justified third-line treatment option in AIH [78].
Belimumab was first administered as third-line agent in combination with corticosteroids and MMF in 2 patients with advanced fibrosis/cirrhosis and refractory disease. Results were more than encouraging as both patients achieved CBR with histological reversion of cirrhosis in one available liver biopsy while both remained in CBR under low-dose corticosteroids, without serious adverse events [79]. Recently, a small case series of 6 patients with AIH and/or primary biliary cholangitis (PBC) and other concomitant autoimmune diseases, treated with belimumab was published [80]. In this retrospective analysis an improvement of liver biochemistry was reported after belimumab initiation along with reduce of corticosteroids doses in patients with AIH, whereas no improvement of liver function tests was observed regarding patients with PBC [80]. However, due to the heterogeneity of the cohort, the co-existence of other autoimmune diseases and the combination of different immunosuppressive agents in each case, these results should be interpreted with caution.
Beyond their limitations, these preliminary findings indicate belimumab as a promising treatment option for patients with AIH and refractory disease. Currently, the effectiveness of lanalumab, another BAFF receptor inhibitor, in treating patients with AIH who are not responding to conventional medication was investigated in a multicentre phase II/III trial (NCT03217422) and results are going to be presented soon.
All patients with a new diagnosis of AIH and mHAI ≥ 4 should receive first-line treatment with corticosteroids and AZA. According to the new data from randomised control, propensity matching, and cohort studies, the administration of corticosteroids with MMF is expected to become the first choice or at least an alternative first-line treatment of AIH patients soon [22, 33–35]. In this case, long term family planning needs to be discussed with the patients at reproductive age before treatment initiation because MMF is definitely contraindicated in pregnancy. Predniso(lo)ne should be started at a dose of 0.5–1 mg/kg per day [1–5]. According to the AASLD guidelines, budesonide at a dose of 9 mg/day could be used instead of predniso(lo)ne for the induction and maintenance of response in patients with low disease activity, but its use is contraindicated in patients with acute severe course of the disease or established cirrhosis [2, 4, 5, 81]. However, a very recent, large real-world study showed that budesonide as first-line treatment was inferior to standard predniso(lo)ne administration as attested by the significantly lower CBR rates at 6- and 12-months after treatment initiation and during follow-up as well as lower probability of CBR and similar side effects after adjustment for cirrhosis and acute severe form of the disease [82] (Figure 1).
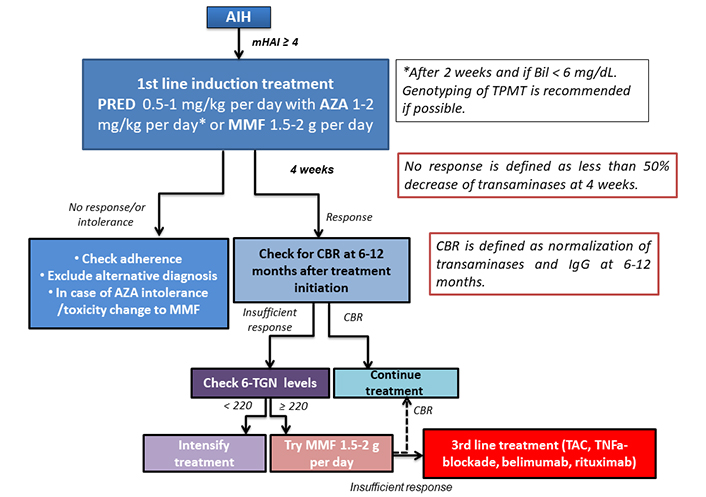
Algorithm of AIH treatment. Bil: bilirubin; PRED: prednisolone; TPMT: thiopurine S-methyltransferase
AZA at a dose of 1–2 mg/kg per day should be initiated preferably after 2 weeks and only if bilirubin levels are lower than 6 mg/dL (Figure 1). Genotyping of TPMT, the main enzyme interfering in AZA metabolism could ideally be performed before AZA initiation as patients with low TPMT activity are at increased risk of AZA toxicity, but it is not widely available [2, 4]. For patients with AZA intolerance, principally due to gastrointestinal symptoms, 6-MP could be tried even though the evidence is very weak. Alternatively, MMF could be initiated at a dose of 1.5–2 g/day (Figure 1).
At 4 weeks and 6 months after treatment initiation, its effectiveness should be assessed (non-response and CBR, respectively) [14]. Alternative diagnoses and problems of adherence to the treatment schedule should be ruled out in situations of non-response at 4 weeks, which is defined by failure to reduce transaminases more than 50% from baseline levels [14]. In insufficient response which is defined as lack of CBR no later than 6 months, determination of AZA metabolites, 6-TGN, and 6-methyl-mercaptopourine (6-MMP) is recommended [24]. Low 6-TGN levels (< 220 pmol/8 × 108 red blood cells) suggest low adherence, but in case of adequate treatment adherence, AZA dose needs to be adjusted [24]. In patients with 6-TGN ≥ 220 and insufficient response, MMF could be tried and then a third-line treatment should be initiated according to each centre’s experience (Figure 1). TAC at a dose of 0.1 mg/kg per day (trough levels 6–8 mg/mL) and progressive tapering once CBR has been achieved is a potential alternative, but strict and close follow up is needed because of high frequency of side effects. Otherwise, belimumab (10 mg/kg at days 0 and 14 and every 28 days thereafter according to response) or rituximab at a dose of 1,000 mg on days 0 and 14 with repeated doses after 6–12 months according to response are promising third-line options. Other third-line options are less studied, so treatment decisions depend on the expertise of each centre. Once CBR is achieved all agents should be progressively tapered in order to maintain CBR with the lowest possible doses.
AIH is a chronic liver disease with need for long term immunosuppression. Although most patients achieve CBR to standard of care immunosuppressive therapy with corticosteroids in combination with AZA or MMF, there is still a fair number of patients who will not tolerate or respond to conventional therapy. Although, second-line treatments and principally MMF are safe and effective alternatives for cases of treatment intolerance, their efficacy in patients unresponsive to first-line treatment is considerably lower.
As a result, patients with insufficient response to first- and second-line treatments are exposed to high doses of corticosteroids in the long term, with all subsequent side effects and are at high risk of developing refractory disease which can progressively lead to the development of cirrhosis and end-stage liver disease. For all these reasons safe and effective third-line treatments in AIH are urgently warranted. Unfortunately, all available data from the current literature on this topic are extremely limited, including small and heterogeneous cohorts, while treatment regimens used are principally based on the expertise of each centre. Therefore, it is clear that multicentre prospective trials are warranted in order to investigate the role of different third-line treatment options in difficult to treat patients with AIH.
AIH lies behind other autoimmune disease as far as novel treatment regimens are concerned. Hopefully the last years, although limited, a number of phase II/III trials are on the way to test the efficacy of new treatment agents. A phase II/III randomized placebo-controlled study (NCT03217422), started in 2018, has tested the use of VAY736 (lanalumab), a BAFF blockade, in patient’s intolerant or unresponsive to standard of care and primary results are expected to be presented soon. Accordingly, JKB-122, a Toll like receptor-4 (TLR4) antagonist previously proven to attenuate liver inflammation in AIH animal models is the target of a phase II trial (NCT04371718). Patients with refractory disease will receive JKB-122 in combination with standard of care treatment and its efficacy will be tested at both the biochemical and histological level. Following the results of a small case-study [83], patients with AIH were included in a phase II open label study evaluating the effect of low doses of interleukin-2 (IL-2) in diverse autoimmune diseases (NCT01988506), inducing the through response of T regulatory cells. Last but not least, zetomipzomib a selective immunoproteasome inhibitor is being tested in a phase IIa trial (NCT05569759) designed for checking its efficacy in patients with insufficient response to first-line treatment. Considering the abovementioned data, the future in AIH seems to be rather promising, but more efforts and new ideas in the field are warranted. The new data on MMF efficacy could further enhance these encouraging efforts.
6-MP: 6-mercaptopurine
6-TGN: 6-thioguanine nucleotides
AASLD: American Association for the Study of Liver Diseases
AIH: autoimmune hepatitis
AZA: azathioprine
BAFF: B cell activating factor
CBR: complete biochemical response
DILI: drug induced liver injury
IAIHG: International Autoimmune Hepatitis Group
IgG: immunoglobulin G
MMF: mycophenolate mofetil
MTX: methotrexate
TAC: tacrolimus
TPMT: thiopurine S-methyltransferase
PA: Investigation, Writing—original draft, Writing—review & editing. IO, SRT, GND, and MCL: Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3403
Download: 22
Times Cited: 0
