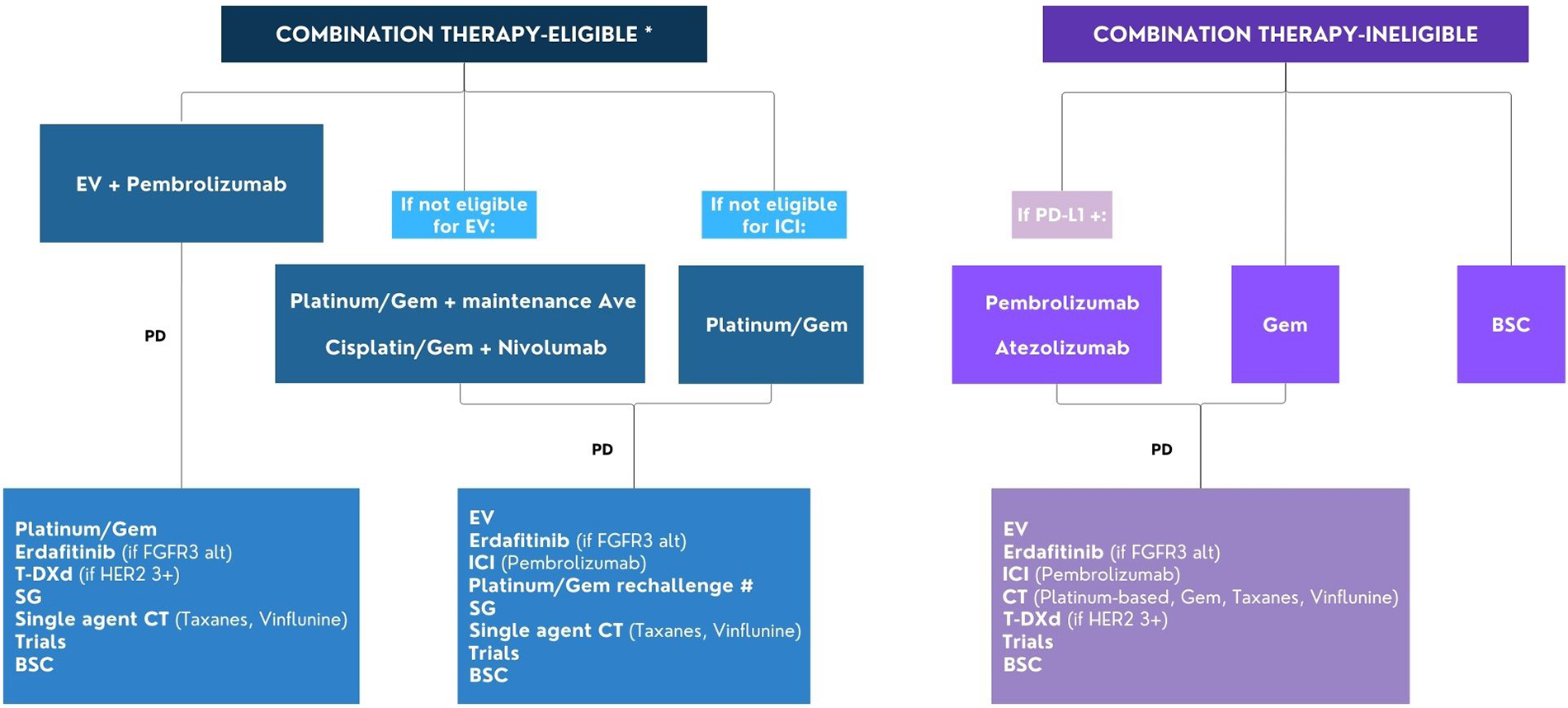
Flow chart for the management of patients with advanced/metastatic urothelial cancer. * Combination therapy eligibility: ECOG PS 0-2, GFR ≥ 30 ml/min, adequate organ functions; # Rechallenge with platinum/Gem if progression occurred ≥ 12 months after the end of previous platinum-based CT or ≥ 12 months after the end of previous platinum-based CT and maintenance avelumab. Alt: alterations; Ave: avelumab; BSC: best supportive care; CT: chemotherapy; EV: enfortumab vedotin; FGFR: fibroblast growth factor receptor; Gem: gemcitabine; ICI: immune checkpoint inhibitor; PD: progression disease; PD-L1: programmed cell death ligand-1; SG: sacituzumab govitecan; T-DXd: trastuzumab deruxtecan; ECOG PS: Eastern Cooperative Oncology Group Performance Status. This image was created using Canva, https://www.canva.com/